WITH THE COLLABORATION OF:
Francesco Guarnieri,
MD, Clinica
GUARNIERI - Rome
Enrico Nicolò, MD, McKeesport Hospital -
Pittsburgh,
Pennsylvania USA
Drawings by Mariacarla Santorelli.
INDEX
The external oblique aponeurosis. The cribriform fascia. The internal oblique muscle. The transversus muscle. The aponeurosis of the transversus muscle and transversalis fascia The deep inguinal ring. The spermatic cord. The preperitoneal tissue and peritoneum. The vessels. The nerves. The femoral canal and the Cooper ligament. References
The Inguinal approach. The preperitoneal approach. The laparoscopic approach. Comments. References.
Isolation of the sac. Resection of the sac. Abandonment of the sac. Comments. References
4. Repair techniques through direct sutures
The Bassini Repair technique. The Postempski or Halsted Repair. The McVay Repair. The Shouldice Repair. The Marcy Repair. References.
The Rives technique. Lichtenstein’s "tension free" hernioplasty. The sutureless "Mesh-Plug" technique. The Stoppa technique (with giant extraperitoneal mesh). The Wantz preperitoneal technique. The
Nyhus technique. Laparoscopic hernioplasty. References.
PART TWO – PHYSIOLOGICAL HERNIOPLASTY
6. A re-examination of the inguinal
region from an
anatomical and functional point of view
The
anatomic and functional aspects of the anterior abdominal wall. The structural aspects of the main anatomical
layers of
the inguinal region in normal conditions and in hernia patients. The normal defense mechanisms of the inguinal region
(sling,
sphincter and shutter mechanisms). The
functional
aspects of the inguinal region in hernia patients. Deductions. The myopectineal
orifice.
8. Physiological hernioplasty
The technique. The suture materials.
The main technical details.
9. The use of meshes
The use of preperitoneal meshes in primary hernia.
The use of meshes in the prefascial area in
primary
hernia. The use of meshes in large inguinal
and
crural hernias. The use of the meshes in
crural
hernia. The Locked-Plug technique. The use of
meshes in inguinal recurrences.
10. Cases and results
Primary direct and indirect hernias. Recurrent hernias. Locked-Plug.
Follow-up.
11. Rationale
The
elimination
of the deep and formation of the new ring. The
narrowing
and shortening of the inguinal canal. Overlapping
the
external oblique aponeurotic flaps. Preservation
of the
cremaster. Discussion. References
12. The "Sandwich" technique in incisional
hernias
Incisions. Treatment
of
the sac. "Sandwich" Repair.
INTRODUCTION
Widespread and easily tolerated, the inguinal hernia is seen as a
minor
disorder. Because hernia surgery may be performed easily and
successfully in
both in- and out-patient environments it is too often dismissed as a
trivial
complaint. On the other hand, in many countries it is considered a
specialization.
Unless inguinal hernia is treated properly, in fact,
it may
turn out to be very disabling. Furthermore, international statistics
show that
recurrences exceed the 10% mark. This means high social costs. In Italy
the
number of hernia operations per annum stands around 100,000. Recurrent
hernia
surgery presents a higher relapse risk rate than primary surgery.
Repeated
operations may also represent a hazard for the testicular vessels.
The
fact
that the solution to the problem is by no means straightforward is
reflected in
the existence of about 80 techniques, of which over 20 currently in use.
Modern hernia surgery came to the fore in Italy in 1884 with
Edoardo
Bassini. His technique, based on the reconstruction of normal anatomical
conditions, is one of the most frequently performed techniques in use
even
today, perhaps because it is easy to carry out despite its limits. It
eliminates
the physiological mechanisms that defend the inguinal region from the
stress of
endoabdominal pressure and creates a cicatricial barrier. However, in
large
hernias, excess suture traction remains and the risk of recurrence is
high.
Prosthetic surgery. Since the end of the 1950's,
biocompatible meshes
have provided hernia surgery with noteworthy advantages. The primary
benefit of
prosthetic surgery is that weak tissue is replaced and suture tension
eliminated. Although many surgeons advocate the employment of prosthetic
meshes,
they have not yet been universally accepted. Effectively speaking,
the use
of foreign bodies, that is, meshes in all hernias does appear too much
of an
exaggeration.
Physiological hernioplasty is the name I have
given to
the technique I outline here. At the end of the 1980's, having used
various
techniques, with and without mesh, I grew dissatisfied with the
cicatricial
barrier produced by traditional techniques and with overuse of
prostheses. So, I
began to seek a new solution.
My primary goal was to reconstruct
the
physiology by reactivating the inguinal region’s muscular defense
mechanisms. The inguinal region is a notoriously weak area because it is
crossed
by the tunnel containing the spermatic cord running through prevalently
fascial
tissue. On the contrary, where muscle tissue exists, there is no hernia
because
this tissue contracts and hardens when endoabdominal pressure increases.
In
hernia patients the muscles of the inguinal region are nearly always
hypotrophic
and the inguinal canal altered. I thought of the possibility of
modifying the
anatomic structure of the inguinal region so that it might be adapted to
the
needs of physiology. It was clear to me that any attempt at
repairing
the deep ring would be a failure because the tissue surrounding it is
particularly weak in hernia patients. Therefore, I thought of closing
the ring
completely and creating a totally new one at the same anatomic level,
but more
medial than the original and in a stronger area. At the same time, it
occurred
to me that the external oblique aponeurosis might be exploited as an
extraordinarily efficacious biological "prosthesis" to reinforce the
non-muscle
zones and modify the dimensions of the inguinal canal which could then
be
adjusted to the muscular-tissue. In December 1988 I began to make use of
this
method. Since then, we have operated on over 2,000 inguinal hernia
patients.
The results have been successful. The incidence of recurrence stands
at
about 0.7% for primary hernias and most of the operations have been
performed in
local anesthesia. The meshes used as reinforcement in primary hernias,
were
availed of only in the presence of very poor tissues, that is, about 5%
of the
total for the experienced surgeon.
All the research and clinical
work has
been carried out in two private hospital departments: the CLINICA
GUARNIERI and
ARS MEDICA in Rome. This is rather unusual for Italy where most research
is
carried out in public hospitals and in university clinics.
I wish to
thank
all my collaborators: doctors, technicians, and my team of nurses,
clerks and
assistants. They are all wonderful people indeed.
This book is not a
new
edition of my previous "La nuova chirurgia dell'ernia"
(Masson
1995) but rather a condensed and updated version of it.
It is
intended for
the surgeons of today but above all for those of tomorrow, who, maybe
when
enthusiasm for prostheses dies down and old methods grow even older,
will judge
my proposal with greater serenity and equilibrium.
Rome, August 1999
ANTONIO GUARNIERI
Part One - Current Techniques
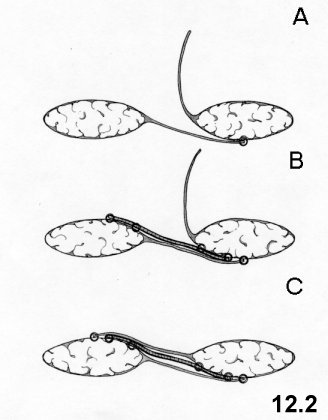
The external oblique aponeurosis (Fig. 1.1)
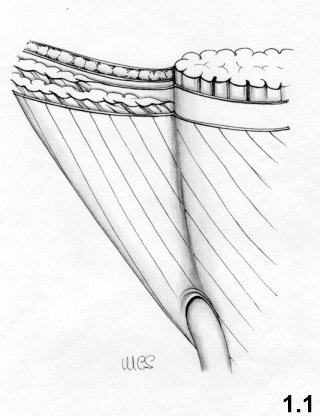
The external oblique aponeurosis is the front wall of the inguinal
canal and,
at its lateral and lower location, is the continuum of the inguinal
ligament.
The superficial inguinal ring is the passage through which the spermatic
cord
passes and is covered by a thin membrane - the external spermatic
fascia. The
external oblique aponeurosis is joined medially to the aponeurosis of
the
internal oblique and transversus muscles, forming the medial half of the
anterior rectus sheath (Fig. 1.3).
The
lateral half of the rectus sheath is simply covered by the external
oblique
aponeurosis, from which it may be separated with greater or lesser ease.
Contraction of the external oblique muscle stiffens the aponeurosis
and
causes a narrowing of the superficial ring.
This is a thin layer that occludes the fossa ovalis. It is the continuation of the femoralis fascia and is joined to the external oblique aponeurosis. It covers the femoral canal from which it is separated by lax fatty tissue.
The internal oblique muscle (Fig. 1.2)

Below the external oblique aponeurosis lies a lower layer. Medially,
it
consists of the lateral side of the rectus sheath; originating from the
fusion
of the aponeurosis of the internal oblique muscle and the transversus
muscle.
Continuing laterally we find the internal oblique muscle which usually
borders
on the rectus sheath and sometimes covers it completely.
Only the
inferior
part of the internal oblique muscle is a part of the inguinal region. It
covers
the transversus muscle and its aponeurosis. The lower fibers of the
internal
oblique muscle form an arch that circumscribes the funiculus along the
inguinal
canal. The inferior border of the internal oblique muscle normally
reaches the
pubic spine. In hernia patients, the insertion of the inferior edge of
the
internal oblique muscle often reaches the rectus sheath in a position
rather
high compared to the pubic spine. The result is a triangular zone
surrounded by
the inferior border of the internal oblique muscle, by the inguinal
ligament and
by the lateral border of the rectus sheath. Thus, this area, called the
inguinal triangle (see Fig. 6.3), is not defended by the internal oblique muscle, which
gives rise
to a tendency to yield and produce direct hernias. The inguinal
triangle must
not be confused with the Hesselbach triangle which is surrounded by
the
inguinal ligament, inferior epigastric vessels and the lateral border of
the
rectus muscle.
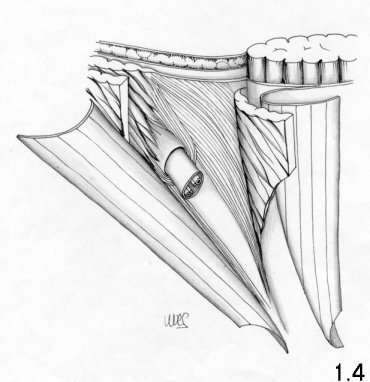
The transversus muscle (Fig. 1.3 - 1.4) 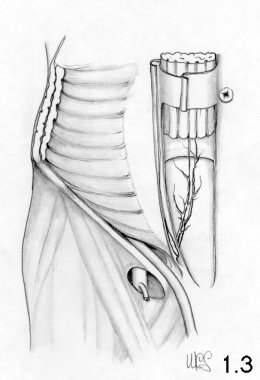
![]()
The transversus muscle follows the same path as the internal oblique muscle, is located deeper and is less present in the inguinal region than the latter. The inferior edge of the muscular part does not, in most cases, reach the midpoint of the inguinal ligament. In 26% of all cases does not go beyond the anterior superior iliac spine. Medially too this muscular portion ends at a certain distance from the rectus muscle. The transversus muscle at inguinal canal level is scarcely represented.
The aponeurosis of the transversus muscle and transversalis
fascia – The
deep inguinal ring (Fig 1.4)
The anterior aponeurosis
of the
transversus muscle and the transversalis fascia are practically joined
together
and represent the posterior plane of the inguinal region. To be exact,
the
aponeurosis of the transversus forms an arch, called the aponeurotic
arch of
the transversus, which coincides substantially with the arch of the
internal
oblique muscle. Therefore, the posterior wall of the canal, behind the
funiculus, consists of a layer, the transversalis fascia, which is
reinforced
laterally by the iliopubic tract and medially by the aponeurotic arch of
the
transversus. The aponeurotic arch of the transversus should not be
confused with
the semilunar line of Spigelio (Fig 1.3) that is,
the
border between the muscular and the aponeurotic part of the transversus
which
runs from the hypochondrium to the inguinal region. Cranially and
laterally, the
deep ring is bordered on by the trasversalis fascia and transversus
muscle or by
its aponeurosis. Medially and caudally, it borders on the plane
comprising the
aponeurosis of the transversus + transversalis fascia, which in this
tract
presents a sling-shaped thickening. The two ends of this thickening are
called,
respectively, inferior and superior crura. The inferior crus is the
shorter of
the two, is positioned laterally, joining the iliopubic tract. The
superior
crus, which is longer, is directed upwards, laterally and backwards,
forming a
flap on the trasversalis fascia to the inner side of the deep
ring.
Medially, the aponeurosis of the transversus muscle joins the
aponeurosis of
the internal oblique muscle to form the anterior part of the rectus
sheath while
the trasversalis fascia passes behind the rectus muscle. Laterally,
along the
angle of the transversalis fascia and the inguinal ligament there is a
thickening, the iliopubic tract. At a deeper level, the
transversalis
fascia joins the femoral vessels and the Cooper ligament, and forms the
femoral
septum that occludes the crural ring.
The contraction of the transversus muscle attracts the
superior crus
upwards and laterally and with it, the fold of the transversalis fascia
which
covers the deep ring from the inside (sling effect) like an eyelid. The
inferior
crus is fixed. The deep ring, besides being covered posteriorly, is
tightened by
the fibers of the aponeurosis of the transversus and pulled upwards and
outwards. When the muscles contract, the deep ring passes under the
internal
oblique muscle, which, is simultaneously tended and lowered. This
protection
mechanism is called the "sphincter mechanism".
The
simultaneous
contraction of the internal oblique and transverse muscles creates the
Keith
shutter mechanism, which protects the posterior wall of the inguinal
canal from
endoabdominal pressure As a result of the contraction, the internal
oblique
muscle stiffens and becomes shorter; the arch straightens, lowers and
leans on
the inguinal ligament. The same happens to the aponeurotic arch of the
transversus muscle.
The most important elements of the spermatic cord are: the
deferent duct,
deferential artery; the testicular artery; the pampiniform plexus. These
elements are enveloped by the internal spermatic fascia, which forms a
continuum
with the transversalis fascia. Externally, we find the cremaster. The
cremaster
is the continuum of the internal oblique muscle and pulls the testicle
up
towards the superficial inguinal ring. The genital branch of the
genitofemoral
nerve innervates it. It is vascularized by the funicular artery, a
branch of the
inferior epigastric artery.
In women, the content of the inguinal
canal is
the round ligament, accompanied by some unimportant vessels (artery of
the round
ligament) and by nerves (iliohypogastric, ilioinguinal, and
genitofemoral).
The preperitoneal tissue and the peritoneum
The preperitoneal tissue is mostly fat and is located between the transversalis fascia and the peritoneum. It is easily separable from the transversalis fascia.
The inferior epigastric vessels, (artery and two veins) stem from
external
iliac vessels. They pass by the deep inguinal ring, below and medially
with
respect to it, and proceed obliquely towards the posterior surface of
the rectus
muscle. The vessels are located between the peritoneum and the
transversalis
fascia. At times they adhere to the transversalis fascia. It is
advisable not
to transect and tie the inferior epigastric vessels, but in cases
of
hemorrhage or when a prosthesis has to be positioned, this may be done
with the
utmost tranquillity.
The funicular vessels stem from inferior
epigastric
vessels and reach the funiculus through the deep ring or a small hole
directly
under this coming very close to the transversalis fascia.
The iliac
and
femoral vessels pass through the lacuna vasorum. They are easily
recognizable in
laparoscopic surgery. In traditional hernia surgery risk of lesion to
these big
vessels is quite remote. But excessive stenosis of a crural hernial
defect
during repair may cause compression of the femoral vein, which is
located
medially to the artery and is often more easily detected through
palpation than
at sight.
The nerves 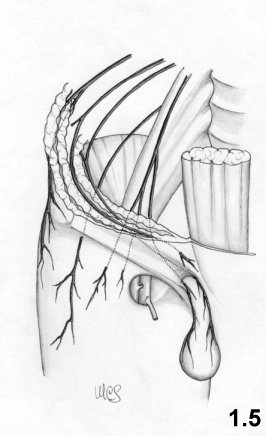
The nerves (Fig. 1.5)
which are greatest
interest are:
- The terminal cutaneous branches of the XI and XII
intercostal
nerves.
- The genital branches of iliohypogastric and
ilioinguinal run parallel to each other. The iliohypogastric nerve
runs
above the ilioinguinal one before turning inwards. At the iliac crest
they pass
between the transversus and the internal oblique muscles. In the
inguinal canal
they are located between the internal oblique muscle and the external
oblique
aponeurosis together with the funiculus.
During hernia surgery, the
subcutaneous terminal branches, which pass through the external oblique
aponeurosis, can sometimes complicate the mobilization of this layer. It
is
necessary to isolate them; if, on account of their position, they run
the risk
of being strained or becoming tangled in the suture they should
be transected to avoid postsurgical pain.
- The lateral
external cutaneous nerve and the femoral branch of the genitofemoral
innervate the skin of the thigh laterally down to the knee as well as
the skin
on the upper part of the "Scarpa's triangle". These are rather marginal
to the
operating area during hernia surgery.
- The genital branch
of the
genitofemoral nerve penetrates the inguinal canal through the deep
ring.
Together with the funicular vessels, it runs posterior to the funiculus
and
innervates the cremaster. It then exits through the superficial ring and
innervates the skin of the scrotum or the major labium as well as the
superomedial part of the thigh.
These nerves are almost all sensory nerves. The only motor nerve
is the
genital branch of the genitofemoral nerve, which innervates the
cremaster.
It is important to know the nerve path well, not only to
perform local
anesthesia, but also because if cut or caught up in the stitches can
cause
hypoesthesia or postoperative pain, respectively. One may say that even
when
these nerves are cut the ensuing, hypoesthesia diminished over time and
is
confined ultimately to small skin areas.
The femoral canal and the Cooper ligament (Fig. 1.6)
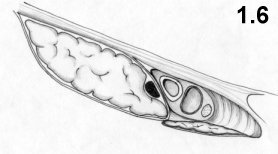
The femoral or crural canal is delimited:
- anteriorly, by the iliopubic tract and immediately to the
front by the
ilioinguinal ligament
- medially, by the Gimbernat ligament
- posteriorly, by the pectineal fascia, which, at level of the
pectineal
line, grows thicker and is called the Cooper ligament
-
laterally, by
the arcus ileopectineus which covers the psoas muscle and separates the
femoral
nerve from the femoral vessels.
Medially to the vein, the femoral canal is closed by the transversalis fascia, which at this point is known as the septum femorale, and is crossed by a number of lymphatic vessels. Crural hernias generally occur medially to the femoral vein, due to weakness in the femoral septum; less frequently prevascular hernias are known to occur.
ReferencesANSON B.J., Mc VAY C.B.: The anatomy of the inguinal
and
hypogastric regions of the abdominal wall. Anat.Rec.70: 211-225,1938.
ANSON B.J., Mc VAY C.B.: Inguinal hernia. The
anatomy of the
region. Surg. Gynecol. Obstet. 66: 186-191, 1938.
CONDON RE.: Surgical anatomy of the transversus abdominis and
transversalis fascia. Ann. Surg. 173:1,1971.
FRUCHAUD
H.: Anatomie chirurgicale des hernies de l'aine. G. DOIN, edit., Paris,
1956.
GLASSOW F.: The Shouldice repair for
inguinal
hernia. In. NYHUS L.M., CONDOM R.E. (Eds): Hernia. J.B. Lippincott Co.,
Philadelphia, 2nd ed., 1978.
HESSELBACH F.C.:
De ortu
herniarum. Werzberg, Stael 1816, cited by LYTLE W.J., Br. J.Surg. 57:
531,
1970
KEITH A.: On the origin and nature of
hernia. Br.
J. Surg. 11:455, 1924
LYTLE W.J.: The
internal inguinal
ring. Br. J. Surg 32: 29, 1945
McVAY C.B.,
ANSON B.J.:
Aponeurotic and fascial continuities in abdomen, pelvis and thigh. Anat.
Rec.
70: 213-231, 1940.
POLJA E.: Die Ursachen der
Rezidive
nach Radikaloperation des Leistenbueche. Zentr. f. Chir. 30: 816, 1912
ROUVIERE H.: Anatomie humaine. Masson, Paris, 1962
RUTLEDGE R.H.: Cooper's ligament repair for adult
groin
hernias. Surgery 87: 601-610, 1980
TESTUT L.,
JACOB O.:
Anatomia topografica. UTET, Torino, 1950
ZIMMERMAN
L.M.: The surgical treatment of direct inguinal hernia. Surg. Gynecol.
Obstet.
66: 192-198, 1938
2 APPROACHES
All modern hernia surgery consists in three phases:
- reaching the sac and the hernia defect
- treating the sac
-
repair
The sac and the hernia defect may be reached through three different surgical approaches: inguinal, preperitoneal and transperitoneal.
The inguinal approach is the most direct. The hernia defect may be reached anteriorly in two ways:
1) through an oblique incision in the skin, parallel to the
groin, and
medially at about a distance of two fingers from it, or
2) by a
transverse
incision at deep inguinal ring level.
The external oblique aponeurosis is incised following the
grain of the
fibers and the superficial ring is opened.
The spermatic cord is
isolated
starting from the pubic spine and drawn back laterally.
In indirect
hernias,
the sac is isolated from the elements of the spermatic cord, once the
internal
spermatic fascia has been opened. In direct hernia, the sac is reached
easily
after cutting the transversalis fascia on the back wall of the inguinal
canal.
The preperitoneal approach
The
hernia defect may be reached from behind through the preperitoneal
space. Today
these approaches have been re-evaluated thanks to the advent of
laparoscopy.
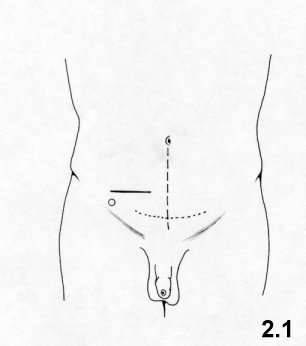
The most common skin incisions currently used are the following (Fig. 2.1):
- midline umbilico – pubic;
- transverse suprapubic according
to the
Pfannenstiel method;
- suprainguinal transversal, two fingers above
the
symphysis pubis.
Dissection of the deep layers
Through a midline incision, passing through the two rectus muscles
the
preperitoneal tissue is reached.
In the Pfannenstiel incision, the
sheath of
the rectus muscles is incised transversally and detached from the
underlying level.
The peritoneum is then separated from its wall
until the
affected inguinal area is reached. The epigastric vessels remain
attached to the
wall.
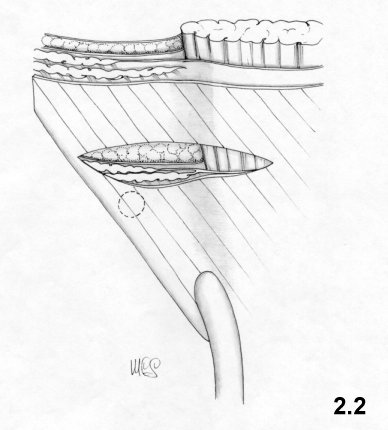
The suprainguinal incision must be executed slightly
above the
deep ring. The incision is made transversally along the rectus sheath
starting
from the midline and across the internal oblique and the transversus
muscles.
This way the transversalis fascia may be reached (Fig.2.2).
The lateral edge of
the rectus muscle is retracted towards the midline. Then the
transversalis
fascia may be incised longitudinally down the lateral edge of the
rectus
muscle or, as Nyhus proposes, transversally, to reduce herniation of the
wound.
Under no circumstances should the peritoneum be cut. This incision leads
to the
inferior epigastric vessels which, normally, must be interrupted and
tied.
Then, continuing to separate the peritoneum from the wall, the
hernial sac
is reached.
The laparoscopic approach
E.Nicolo'
Even if an intraperitoneal
laparoscopic approach exists, a preperitoneal one is generally
preferred.
The preperitoneum may be reached directly, without opening the
peritoneum, as well as transperitoneally.
In the latter case,
the
hernia defect may be reached through the inner side of the abdomen
cavity by an
incision on the parietal peritoneum which will later be sutured.
The
laparoscopic approach requires specific experience and a good "inside"
knowledge
of anatomy.
(See Figs. 5.11 and
5.12).
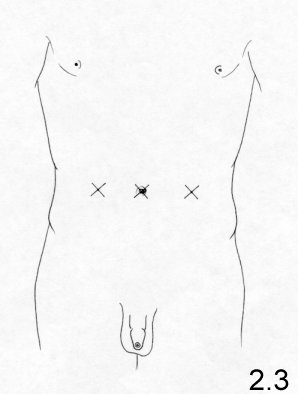
The transabdominal
preperitoneal approach
After having performed a
pneumoperitoneum,
a laparoscope with a 30-degree view is introduced through the umbilicus.
Two
trocars are inserted at the lateral edge of the rectus muscle, one on
the left,
the other on the right, at umbilical level (Fig.
2.3).
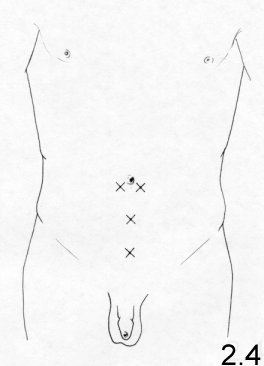
The total
extraperitoneal
approach
A vertical incision, 1-2 cm long, under the
umbilicus
and 1 cm lateral to the linea alba, on the side opposite to the hernia,
is made.
(Fig. 2.4). The anterior
rectus sheath
is incised, the muscle is retracted and a special balloon probe, which
slides
along the posterior sheath of the rectus muscle until it reaches the
pubic bone,
is inserted. The optics are inserted, the balloon is inflated to
separate the
preperitoneum. After 3-4 minutes, the optics are removed. The balloon is
deflated and the probe is removed. Through the same hole, a sealed
trocar is
introduced and carbon dioxide blown in. Two trocars are inserted at the
midline,
one above the pubis, the other half way between the umbilicus and pubis (Fig.2.4).
The inguinal approach
Is undoubtedly the most frequently chosen.
Advantages:
- the possibility of performing under
local
anesthesia
- direct and easy access on all anatomic levels
- very
low
risk of lesion of large vessels.
Disadvantages:
-
difficult
dissection in hernia recurrence with added risk of lesion to testicular
vessels
- frequent traumatism of the inguinal canal nerves with consequent
hypoesthesia and neuralgia
The preperitoneal approach
In many cases this requires a general anesthesia, except in the case of suprainguinal incisions.
Advantages:
- in hernia recurrences, the difficult
dissection of
the scar tissues is avoided. The risk of testicular vessel lesion is
reduced.
- elimination of inguinal canal nerve traumas
- the possibility
of
treating hernia during operation for other pathologies
- bilateral
hernias
may be treated simultaneously if a midline incision is performed
Disadvantages:
- limited possibility of performance in
local
anesthetic
- increased width and depth of the operating field
compared to
the inguinal approach
- impossibility of reaching surface layers of
the
inguinal region
- practically imperative use of prosthesis due to
the poor
results with use of direct suture and avoid risk of hernia on the wound.
The laparoscopic approach
Perhaps, because it is very recent, it is still too soon to
express a proper
evaluation of this new approach and when it is indicated. Problems of
training,
the development of new methods and instruments are being developed. On
the one
hand, enthusiasm for novelty and the strong influence of the biomedical
industry
are keenly felt, but on the other, distrust towards new and more
sophisticated
techniques exists, also because these techniques are difficult to
acquire.
Those who advocate this method assert that the risk of trauma is
low, that
postoperative pain is mild and that immediate resumption of
physical
activity is possible, and that no risk of ischemic orchitis exists. The
criticism this technique arouses is similar to that for extraperitoneal
techniques.
Concluding, the inguinal approach is still
the most
frequently chosen. Only in particular cases are different approaches
preferred.
Cases in which preperitoneal or laparoscopic approaches are
indicated:
-
complicated hernia recurrence and multiple recurrence
- bilateral
hernias to
be treated simultaneously
- treatment of hernia during operations
for other
ailments.
References
CALNE R.Y.: Repair of bilateral hernia, a technique
using
Mersilene mesh behind the rectus abdominis. Br. J. Surg. 54: 917, 1967
CHEATLE G.L.: An operation for the radical cure of
inguinal
and femoral hernia. Br. Med. J. 2: 168.1920
COPELLO
A.J. Technique and results of Teflon mesh repair of complicated
recurrent groin
hernias. Rev. Surg. 25: 95.1968
ESTRIN J. et
al.: The
posterior approach to inguinal and femoral hernia. Surg. Gynecol.
Obstet. 116:
547, 1963.
HENRY A.K.: Operation for femoral
hernia by
a midline extraperitoneal approach. Lancet. 1: 531, 1936
JENNINGS W.K., ANSON B.J.: A new method of repair for
indirect inguinal
hernia considered in reference to parietal anatomy. Surg. Gynecol.
Obstet. 74:
697, 1942
McEVEDY P.G.: Femoral hernia. Ann.
R. Coll.
Surg. Eng. 7: 484, 1950
McNAUGHT G.H.D.:
Femoral
hernia: the rectus sheath operation of McEvedey. J. Coll. Surg. Edinb.
1:309,
1956
MIKKELSEN W.P., BERNE C.J.: Femoral
hernioplasty:
suprapubic extraperitoneal (Cheatle-Henry) approach. Surgery 35: 743,
1954
MOSCHOWITZ A.V.: Femoral hernia: A new
operation
for the radical cure. N.Y State J. Med. 7:396, 1907
MUSGROVE J.E., McCREADY F.J. The Henry approach to femoral
hernia.
Surgery 26: 608, 1949
NYHUS L.M. et al.:
Preperitoneal
herniorrhaphy: A preliminary report in fifty patients. West J. Surg.
Obstet.
Gynecol. 67: 48, 1959
NYTHUS L.M. et al.: The
preperitoneal approach and prosthetic buttress repair for recurrent
hernia: the
evolution of a technique. Ann. Surg. 208: 733-737, 1988
NYTHUS L.M.: Inguinal hernia. Curr. Prob. Surg XXVIII-6:
406-450,
1991
NYTHUS L.M.: The preperitoneal approach
and
iliopubic tract repair of inguinal hernia. In: NYHUS L.M., CONDON R.E.
(eds.):
Hernia. J.B: Lippincott Co., Philadelphia, 3rd. ed., 1989, pp 154 -198
READ R.C.: Preperitoneal exposure. Curr. Prob. Surg.
4. 17,
1967
READ R.C.: Preperitoneal herniorrhaphy: a
historical view. World J. Surg. 13: 532-540, 1989
READ
R.C.: Preperitoneal prosthetic inguinal herniorrhaphy without a relaxing
incision. Am. J. Surg. 132: 749, 1976
REAY-YOUNG
P.S.:
Repair of femoral hernia. Lancet 2: 1217, 1956
STOPPA
R.: et al.: Unsutured Dacron prosthesis in groin hernias. Int. Surg. 60:
411,
1975
WANTZ G.E.: Giant prosthetic
reinforcement of the
visceral sac. Surg. Gynecol. Obstet. 169: 408, 1989
3 TREATMENT OF THE SAC
The hernial sac is an outward bulging of the parietal peritoneum. The sac itself consists of a neck, a body and a fundus. The neck is the proximal portion surrounded by the hernia defect.
For more than a century, the necessity to isolate the sac from
the
transversalis fascia beyond the neck has been known.
Through the
inguinal
approach, the isolation of the sac in direct hernias is quite
straightforward. In indirect hernias, sometimes the sac may reach the
scrotum or
adhere to the funiculus. In these cases wide dissection should not be
performed
because it might provoke distal vein thrombosis and ischemic orchitis:
the sac
may be isolated from the neck up to the pubis and divided at this point.
The
body and the fundus may be left in situ.
The preperitoneal
approach:
in direct hernias the isolation of the sac is again straightforward. In
indirect
hernias, the sac is easily isolated by applying medium traction on the
peritoneum. In case of stubborn adhesions, the sac may be divided at the
level
of the neck and left in situ.
Resection of the sac
Having isolated the sac beyond the neck, the complete resection
and closure,
with ligation or high suture of the sac, are carried out in the
traditional
manner.
Alternatively, after the resection of the sac, the
peritoneal gap
may be left unsutured. Some Authors hold that this does not cause
additional
complications because the peritoneum heals immediately and completely.
Postoperative pain should be less because less phlogosis of the parietal
peritoneum occurs.
Abandonment of the sac
The abandonment of the sac, without even opening it, in the preperitoneal space may be performed in both direct and indirect hernias. Abandonment causes multiple folding of the walls and an effective elimination of the sac, which will not expand.
Personally, I prefer the abandonment of the sac in the
preperitoneum, a
practice which is possible in most cases. I tend to avoid ligations
when
the sac has to be divided to prevent traumatic separation of the body
and
fundus.
Abandonment of the sac, which I have performed during
thousands of
operations, is easy and safe because there is no risk of viscera lesion,
which
may occur in cases of resection. When this not too rare kind of viscera
lesion
occurs, it usually involves the bladder. In any case, the opening of a
sac with
thick walls and /or in the presence of sliding hernia may create
problems.
Another advantage related to sac abandonment, is that postoperative pain
is
reduced noticeably.
FERGUSON D.J.: Closure of the hernial sac. Pro and
Con. In
NYHUS L.M., CONDOM R.E. (eds): HERNIA. 2nd ed., J.B. Lippincott Co.,
Philadelphia, 1978, pp. 152-153
WANTZ G.E.:
Testicular
atrophy as a risk of inguinal hernioplasty. Surg. Gynecol. Obstet. 154:
570-571,
1982
SHULMAN A.G. AMID P.K: LICHTENSTEIN
I.L.: Ligation
of hernial sac a needless step in adult hernioplasty. Int. Surg. 78:
152-153,
1993
SMERDGERG S.G.G., BROOME A.E.A., GULLMO
A:
Ligation of the hernial sac? Surg. Cl. North Am. 64: 299-305, 1984
4 REPAIR TECHNIQUES THROUGH DIRECT SUTURE
Repair may be performed either by suturing the anatomic layers
(herniorrhaphy) or by inserting a biocompatible mesh in order to
reinforce the
tissues (prosthetic hernioplasty). The tissues themselves may be used
for the
same purpose (hernioplasty).
The Bassini, Postempski, McVay,
Shouldice
and Marcy techniques are all performed availing of the inguinal
approach and
are the most frequently used at present.
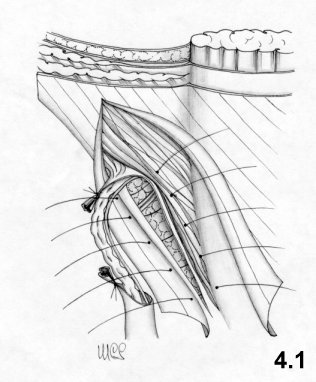
In order to perform the Bassini technique correctly, the
resection of the
cremaster and an incision of the transversalis fascia from the deep
inguinal
ring to the pubic spine are mandatory. These phases are often, omitted,
and
wrongly so. The repair of the inguinal canal takes place upon two
planes. The
deep layers are sutured using separate stitches, one centimeter apart.
The
suture starts from the pubis and medially includes three layers: the
internal
oblique muscle, the aponeurosis of the transversus muscle and
transversalis
fascia; laterally, the iliopubic tract and the inguinal ligament (Fig. 4.1). The suture
reaches deep
ring, which is tightened in such a way as to avoid compression of the
cord
vessels.
Once the funiculus is placed to the front of this suture,
the
external oblique aponeurosis is sutured.
At lower level, the "joined
tendon"
is not always well represented, in this case the first stitches are
placed on
the rectus sheath.
Bassini's intention was to reconstruct the normal anatomy. Mistakenly, he believed it would also reestablish the normal physiological defense mechanisms, which he believed depended on the obliqueness of the canal and the level variation between the superficial and deep rings.
Comments
On no other technique has so much been written. Many surgeons continue to believe strongly in this technique, even if the reported results are both good and bad. This ambiguity can be due to incomplete follow-up, good/bad execution of the technique, the experience/inexperience of the surgeons. What is unquestionable, though, is the high incidence of recurrence (about 10%).
The incidence of recurrence may be considered the most noticeable drawback of the Bassini technique, although there are others.
Above all, physiology is not respected; in fact, deep inguinal ring, anchored to the inguinal ligament, loses its mobility and its normal defense mechanisms. The transversus and internal oblique muscles are united, while in normal conditions each of them move independently complementary to the other (sphincter effect).
Moreover, the technique does not follow principles of tissue synthesis:
- the stitches that pass through the entire wall may rupture the tissue and create new hernia defects.
- the muscles are not usually fit for sutures: they rupture easily, lose motility and form scar tissue.
- the suture between the rectus sheath and inguinal ligament, performed when the internal oblique muscle is atrophic, is under strong traction due to poor tissue elasticity.
Because it is known and performed worldwide, the advantages of the Bassini technique are that is easily performed and learnt.
The Postempski or Halsted Repair
This method differs from that of Bassini in one way: the repair
of the
external oblique aponeurosis occurs behind the funiculus. The
superficial ring
is located upwards and aligned with the deep ring. The funiculus is made
to run
through the subcutaneous tissue.
Principles of the technique
This
technique aims at eliminating the weak point in the Bassini technique
(the
inferior area) and at creating a scar wall formed by the fusion of the
posterior
and anterior layers.
Comments
This technique creates a
reliable
reinforcement of the weak zone near the pubic spine but creates
alignment
between two weak points: the superficial and deep ring. This alignment
has been
criticized because it eliminates the defense of the external oblique
aponeurosis
on the deep ring, already deprived of the sphincter effect.
Nevertheless, the
incidence of recurrence is lower in Postempski’s technique than in
Bassini's.
The recurrences of direct hernia at the inferior angle are very rare,
while
those of indirect hernia are the same as in Bassini. These results are
obvious,
since the external oblique aponeurosis supports the levels below. The
risk for
recurrence is linked to the resistance of the deep layers and to the
deterioration of the physiologic defense mechanisms.
This technique is in keeping with current inguinal and crural
hernia therapy
and with the supporters of the Fruchaud thesis on the need (on
principle) to
treat the myopectineal orifice. Lotheissen devised it in 1897, but
without
practicing a relaxing incision on the rectus sheath; the suture traction
was
excessive.
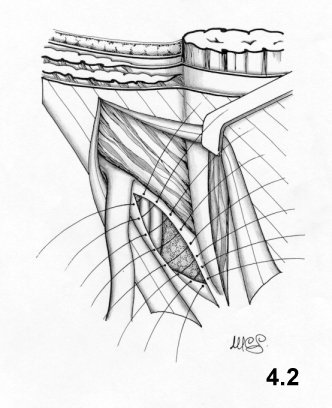
The transversalis fascia must be opened as far as the
pubis to
reach the Cooper ligament. Repair occurs on two layers. The deep layer
is
created, using interrupted stitches to join the transversus aponeurosis +
transversalis to the Cooper's ligament as far as the femoral vein, which
should
not be compressed. Then, the aponeurotic layer is sutured to the femoral
sheath
and to the iliopubic tract as far as the deep ring. A relaxing incision
on the
rectus sheath is performed in advance to avoid excessive suture tension.
(Fig. 4.2). The inguinal
ligament and the
internal oblique muscle are not involved in the suture.
The
funiculus is
relocated on this layer and the external oblique aponeurosis sutured.
This technique aims at anatomical repair of the whole myopectineal orifice: in fact the Cooper ligament is considered to be the perfect continuation of the transversalis fascia.
Comments
This technique respects both anatomy and physiology, because it does not compromise the motility of the deep ring and internal oblique muscle. But the repair of the deep ring does not guarantee solidity, therefore an indirect hernia may form. Frequently, repair is not well performed because the transversalis fascia near the deep ring is often dystrophic and very thin. The suture between the transversalis fascia and aponeurosis of the transversus muscle and the Cooper ligament seems unreliable, because it may come under tension. Also, the thin aponeurotic layer, if not protected sufficiently by the internal oblique muscle, may yield.
Nevertheless, this technique proves more successful than Bassini's. The incidence of recurrence has shown to vary from 3.5% to 7.5%. I think that these results are linked to the fact that physiology is respected and that the rectus muscle may expand laterally. As a consequence, the weak zone is reduced.
Shouldice's technique was devised between 1945 and 1953
optimizing the
Bassini technique. For example, suturing with an overlapping of the
transversalis fascia (Harrison 1922).
Resection of the cremaster and
opening
of the transversalis fascia from the deep ring to the pubic spine are
mandatory
as well as systematic exploration of the crural ring. Repair is
performed with
three continuous doubleline ("back and forth") sutures.
The first
retrofunicular suture line joins the inner surface of the transversalis
fascia
(close to the lateral margin of the rectus muscle) to the iliopubic
tract,
beginning from the pubic spine up to the deep ring. The suture reaches
the deep
ring, and includes the proximal stump of the cremaster in order to
repair and
reinforce the ring (Fig. 4.3). On its way back, the second suture line joins the
medial flap
of the transversalis fascia, left over by the previous step, to the
inguinal
ligament. In this way, a suture with overlapping flaps of the
transversalis
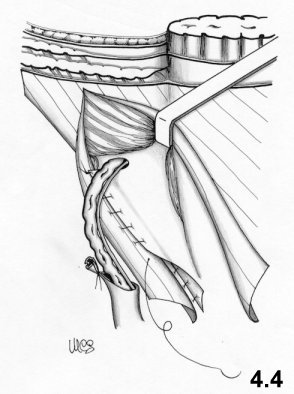
fascia is obtained (Fig. 4.4).
The
second retrofunicular suture line, on the way out, joins the margin of
the
internal oblique muscle to the inguinal ligament near the previous level
and on
the way back includes the anterior surface of the internal oblique
muscle and
the inner surface of the lateral flap of the external oblique
aponeurosis. Once
the suture has been performed, the distal stump of the cremaster is also
included to sustain the testicle. 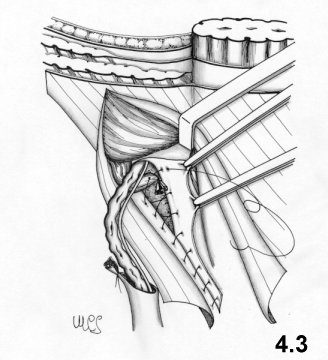
![]()
The
funiculus is then replaced at this
level.A third doubleline continuous suture passes in front of the
funiculus
and joins the margin of the lateral flap of the external oblique
aponeurosis and
the inner surface of its medial flap 2-3 cm from the border. As it
returns, the
medial flap covers and is sutured to the lateral one. The original
technique
requires the use of steel thread.
Some changes have been performed
to this
technique: the abolition of the third and fourth layer and the repair
without
overlapping of the external oblique aponeurosis flaps. The results do
not seem
equally encouraging.
The principles of the
technique
A series of improvements and changes concerning the
tissue-synthesis have improved the Bassini technique:
- no suture tension occurs because the rectus muscle is mobilized.
- sutures are not aligned and do not involve the whole wall.
- scar surfaces rather than scar borders are produced
- the modeling of the deep ring, using the proximal stump of the cremaster, is improved
It should be observed that the second suture layer blocks the deep ring on to the inguinal ligament, while the third and fourth layers involve the internal oblique muscle completely. This means that physiology is not respected at all.
On the other hand, tissue-synthesis is respected and an accurate repair of the deep inguinal ring is achieved.
Results proved to be good. The incidence of postoperative recurrence is lower than 1% in cases performed by the surgeons from the Shouldice Clinic. According to them, to obtain these results, a five-year training period is necessary.
The Marcy technique was published in 1871. Although a century
old, it still
shows interesting characteristics although limited in scope.
This
technique
implies the resection of the cremaster and a careful exposition of the
deep
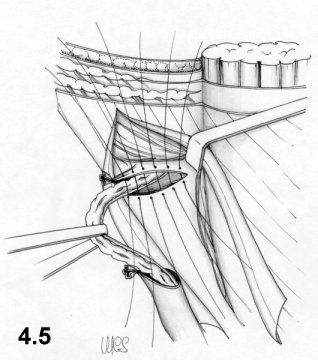
ring. Having treated the sac, the ring is repaired and calibrated with a
suture
which medially recomposes the transversalis fascia and the
transversus aponeurotic layer (Fig. 4.5). A simple suture then repairs the external oblique
aponeurosis.
This repair technique respects the normal anatomy and physiology of the inguinal canal.
Comments
This technique’s major defect is its lack of treatment of the posterior wall of the inguinal canal.
This technique is suitable for indirect hernias and when both the internal oblique muscle and the transversalis fascia are in good condition, as forms of congenital hernias in babies and youths. In such cases, in fact, resection of the cremaster is not the best choice.
Surgeons very familiar with the deep ring may carry out satisfactory repair availing of the Marcy technique without resecting the cremaster; it suffices to separate the elements of the funiculus from the proximal tract of the cremaster. References
ASMUSSEN T., JENSEN F.U.: A follow-up study on
recurrence after
inguinal hernia repair. Surg. Gynecol. Obstet 156: 198-200. 1983
BARBIER J. et al: Traitment des hernies inguinales
selon la
technique de Mc Vay. A propos de 1000 cas. Chirurgie 110: 144, 1984
BASSINI E.: Nuovo metodo operativo per la cura
dell'ernia
inguinale. Padova, 1889.
BERLINER S.D., WISE
L.,
Transversalis fascia hernioplasty. N.Y. State J. Med. 80: 25-27, 1980
GLASSOW F.: The surgical repair of inguinal and
femoral
hernias. Can. Med. Assoc. J. 108: 308-313, 1973
GRIFFIT
C.A.: The Marcy repair of indirect inguinal hernia. In: NYHUS L.M.,
CONDON R.E.
(eds): Hernia. Edition 2. J.B. Lippincott Co. Philadelphia, 1978
HALVERSON K. McVAY C.B.: Inguinal and femoral
hernioplasty. A
22 years study of the Author's method. Arch. Surg. 101: 127-135, 1970
ILES J.D.H.: Specialization in elective
herniorrhaphy Lancet
1: 751-755, 1965
Mc VAY C.B.: Inguinal and
femoral
hernioplasty: Anatomic repair. Arch. Surg 57: 524-530, 1948
TONS C. et al.: Cremaster resection in Shouldice repair. A
prospective
controlled bicenter study. Chirurg. 61(2): 109-111, 1990
5 MESH REPAIR
Mesh repair is not a feature of traditional methods, because the
materials
available before polypropylene were inappropriate. From the beginning of
the
20th century, numerous techniques using metal mesh or
tissue-implants
were devised to solve the problem of defects in large hernias, but the
results
were unacceptable.
At the end of the 1950's, meshes made of plastic
materials and well tolerated by the tissues, were introduced.
Preperitoneal
approaches flourished again and particular attention was paid to
traditional
methods, which were then improved. New techniques which made meshes a
focal
feature, even in the treatment of primary hernias, were devised.
According those
who advocate meshes, these should be used in all cases, because they
avoid
suture tension completely and reduce the incidence of recurrence
considerably.
Today, the most frequently used meshes are those made of
polypropylene,
Dacron and PTFE.
Current techniques position meshes in the
preperitoneum or
between the intermediate layer (internal oblique muscle and aponeurosis
of the
transversus) and the external oblique aponeurosis.
As in traditional
methods, the approach may be inguinal, preperitoneal or laparoscopic.
Only
the most widely performed techniques will be discussed here.
The Rives technique
This
technique was created in 1965. The approach is inguinal. The cremaster
is
sectioned near the deep ring. The transversalis fascia is incised
along the
inguinal canal, so that the Cooper ligament is exposed. A preshaped (10
x10 cm)
mesh of Dacron with a curved lacuna for the passage of the iliac
vessels, is
fixed onto the Cooper ligament using 4-5 stitches along the
approximately 3-cm
hem of the inferior flap. The flap is positioned behind the iliopubic
branch to
increase the contact surface. The medial flap of the mesh is fixed on
the deep
surface of the wide muscles by means of a series of U-shaped stitches
that
penetrate the intermediate layer. A cut is performed on the
superoexternal side
of the mesh as far as deep ring layer to allow for the passage of the
funiculus
(Fig. 5.1). The
flaps of the mesh are sutured to the wall using more U-shaped stitches,
to form
a ring, positioned as high up as possible and calibrated around the
funiculus.
At its inferior-external border the mesh is sutured to the vascular
sheath and
to the inguinal ligament. Then the surplus mesh is removed along the
superoexternal side. The transversalis fascia sutured onto the
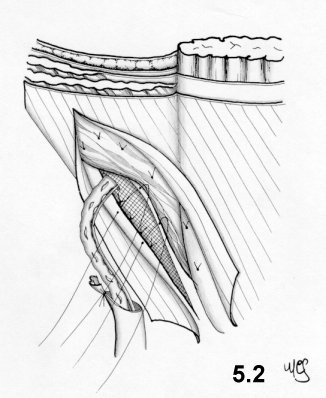
prosthesis (Fig. 5.2). The
funiculus is repositioned
and the external oblique aponeurosis sutured. 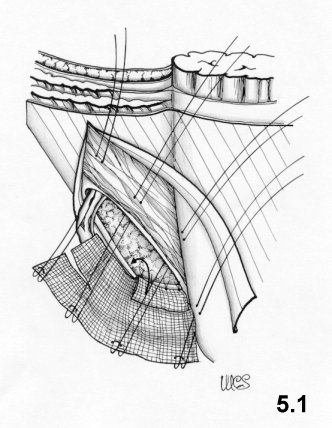
![]()
The
principles of this technique
The principles of this technique are complete treatment of the
myopectineal
orifice and substitution of the transversalis fascia with strong
material.
Comments
The advantage of this technique is that it
requires
neither a large mesh nor major dissections, while anchorage of the mesh
to the
Cooper ligament is strong. The physiology of the inguinal canal is
respected.
The Author, who, while using this technique witnessed a 0.6%
recurrence
rate, recommends it in cases of direct mediumdefect and recurrent
hernias.
I sincerely retain this technique to be efficient. My only doubts
concern the
U-shaped stitches that may cut through tissues and open up new hernial
defects.
Modern laparoscopic surgery, even if it accedes through other
approaches,
uses a mesh anchored to the Cooper ligament and to the wall
to achieve
repairs similar to those obtained by the Rives technique.
Lichtenstein’s "tension-free" hernioplasty
The approach is inguinal. The respect of the iliohypogastric,
ilioinguinal
and genital branch of the genitofemoral nerves is recommended. To
respect the
latter the Author recommends to isolate with the funiculus and to divide
the
cremaster at the level of the internal ring, avoiding to cut the nerve.
The
hernial sac is sent inwards without ties. The external oblique
aponeurosis is
separated from the level below, on which a mesh of polypropylene is
positioned.
An 8 x 16cm spindle shape mesh is cut to fit the inguinal area.
The
procedure starts at the inferior-medial angle: the mesh has to cover
completely
and exceed the pubic spine, then, it is sutured on the fascial tissue,
which
covers and surrounds the bone without including the periosteum. This
suture runs
between the margin of the mesh and the inguinal canal to the level of
the deep
ring . The border of the superolateral mesh is cut to create two flaps: a
wider
superomedial one (2/3) and a narrower inferior-lateral one (1/3) (Fig. 5-3).
The
superomedial flap is passed below the spermatic cord and directed
cranially. The
mesh is stretched under the funiculus and at the level of the deep ring,
which
is located between two flaps.
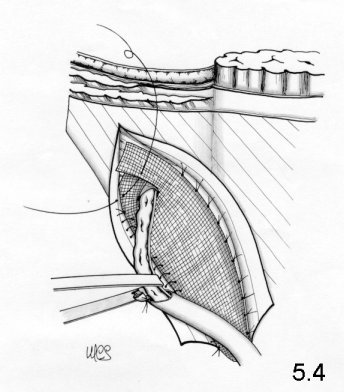
The medial margin of the mesh is
sutured on
the rectus sheath, the superomedial one is put over the inferior lateral
one, to
circumscribe the funiculus. The two flaps, overlapping one another, are
sutured
together with one stitch at the inguinal ligament, immediately above the
deep
ring (Fig.5-4).
Then,
the
mesh is cut to eliminate the surplus, 3-4 cm above the deep ring. The
external
oblique aponeurosis is sutured. 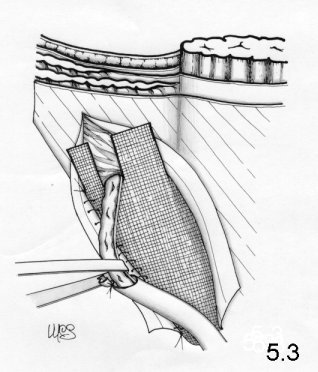
![]()
Principles
of the technique
The Author, a
strong supporter of prostheses (polypropylene and monofilament), trusts
in the
findings of many studies regarding metabolic collagen disorders in
adults
affected by hernia and speaks of the low trustworthiness of tissues
lacking in
collagen fiber. He also believes that suture tension should be avoided.
Comments
Much can be said about the lack of collagen.
This may be
due to a reduction in solicitation of the aponeurosis resulting from
muscular
weakening. Less strength means less solicitation. Moreover, the
excellent
results obtained by the Shouldice technique disprove the theory that
"collagenlow" tissue is unreliable.
As regards the so-called
"tension
free" techniques (an intriguing and exciting slogan) I would like to
make two
observations:
- the absence of tension occurs only at rest, with
very slight
endoabdominal pressure and a loosened wall. But in the erect position
and under
strain, tension spreads uniformly to the whole abdominal wall.
- On a
non-contractile surface (passive area), the solicitation due to an
increase
of the endoabdominal pressure, causes what I call the "sail effect" and
determines traction on the perimeter of the passive zone proportional to
the
surface itself.
There are still doubts regarding the position of the
mesh on
top of the internal oblique muscle. The posterior wall is, indeed,
reinforced by
the mesh, but it is not "sealed". There is a definite risk of intramural
hernias, even if they are small and clinically irrelevant. Concluding,
physiology is not respected, because the neo- deep ring, made of mesh,
is
anchored to the inguinal ligament and the internal oblique muscle is
entangled
in the scar tissue.
Despite these disputable aspects, the technique
produces
good results. The Author shows a 0.1% recurrence and points out that
specific
experience is not required to obtain good results.
The
sutureless "Mesh-Plug" technique
The approach is inguinal.
The Gilbert technique has inspired many more which differ only in as far as the type of plug and the shape of the mesh positioned in front of the transversalis fascia and internal oblique muscle are concerned.
Robbins and Rutkow suggest other types of plug (conical or preshaped) and perform this technique on all hernias. When the hernial defect is large, a bigger plug is used and is sutured to the edges of the hernial defect to avoid dislocation.
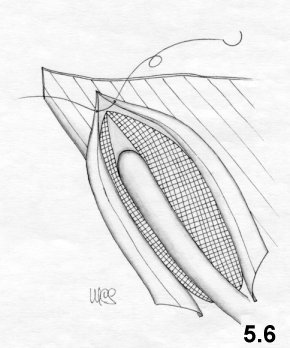
Principles of the technique
Simplicity, rapidity and minimized dissection characterize this technique. According to Gilbert, stitches through the transversalis fascia used to calibrate the deep ring , may distort and weaken the fascia itself, leading to recurrence.
Comments
The most important feature of this technique is its minimization of dissection. No sutures occur to weaken the tissues around the "critical zone", that is the edge of the mesh. Usually this is where greatest solicitation occurs, as shown by the site of recurrences.
It is not true that inexperienced surgeons are in a position to avail of this technique. Hernia treatment requires, in all cases, skill and experience, because, however easy an operation may appear at first sight, it may present sudden and expected difficulties. Repair is not necessarily the most complicated phase of a hernia operation. .
Furthermore I disagree with the use of exceedingly large quantities of mesh as required to make plugs.
The method is presented as physiological and it is in part. However, the mesh positioned in front of the transversalis fascia, provokes a scar reaction capable of entangling the internal oblique muscle, even in the absence of sutures.
The Stoppa technique (with giant extraperitoneal mesh)
Stoppa elaborated this technique on the basis of
a previous
study by Mahorner and Goss (1962), eliminating the stitches used to
anchor the
mesh to the wall.
The approach is preperitoneal through a midline umbilico-pubic
incision. A wide cleavage in the preperitoneal
area is performed, involving the space from the Retzius and bladder to
the
prostate, reaching laterally beyond the inferior epigastric vessels and
below
the rectus muscle to the inguinal region. Once the hernial sac is
reached, it is
isolated by means of moderate traction. If adhesions occur, they should
be
carefully dissected by introducing a finger into the sac itself. Once
the sac is
freed, separation continues downwards to the iliac vessels and laterally
to the
iliac psoas muscle. Then, the testicular vessels are separated as much
as
possible from the peritoneum, so that they adhere to the wall and do not
cross
the preperitoneal space where the mesh will be positioned. At this stage
the
surgeon should stand on the side facing the area to be detached,
although during
the rest of the operation the surgeon stands on the other
side. As
soon as the separation has been carried out, a mesh of Dacron is
prepared. It
should be tailored to fit the patient and corresponds transversally to 2
cm less
than the distance between the anterior-superior iliac spines (about 26
cm) and
vertically to the distance between the umbilicus and pubis (roughly 16
cm). A
very wide V shape is cut into the top and bottom of the mesh (Fig.5.7). 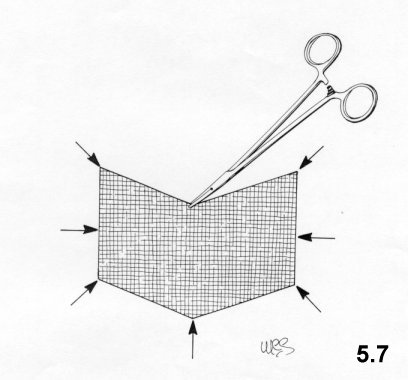
Then, 8
Rochester forceps are positioned at the angles
and in the midpoints of each side of the mesh. The preperitoneal area is
opened
wide and the mesh positioned (Figs. 5.8, 5.9)
using the Rochester forceps.
The central
lower border forceps is inserted between the pubis and bladder, followed
by the
inferolateral forceps, then those positioned at the midpoint of the
lateral
margin and, lastly, those at the superolateral angle. The forceps are
then
pushed as far apart as possible in order to unfold the mesh. They are
then
removed with great care to avoid dislocating the mesh. The same sequence
is
performed on the other side. Again, the surgeon will stand on the side
opposite
the area to be treated. The mesh is fixed to the wall with one stitch
passing
through the upper edge midpoint. The laparotomic wound is then sutured. 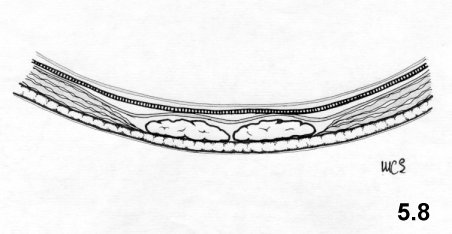
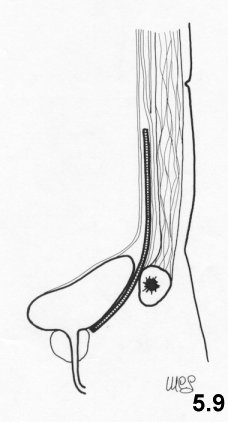
![]()
Principles
of the technique
The giant mesh has the task of surrounding the
visceral
sac and reinforcing the transversalis fascia bilaterally in particular
at
Fruchaud myopectineal orifice level. The mesh is not anchored by
stitches,
because it reaches beyond the hernial defect. According to Pascal's
hydrostatic
principle, it is pushed against the wall by internal-abdominal pressure.
This
pressure is proportional to the surface of the mesh and blocks the
movement.
Comments
This technique respects physiology. The
positioning of
the testicular vessels along the wall avoids creating gaps in the mesh, a
constant source of critical weakness.
It should be underlined: 1)
the amount
of foreign body introduced is considerable. 2) the separation area is so
wide
that this technique cannot possibly be performed in local anesthetic. 3)
that
this kind of surgery requires training. 4) the indications provided are
not
many: plurirecurrent hernias, very large hernias, and bilateral hernias.
It is
in any case a very interesting technique and performed by the Author,
shows a
recurrence rate of 0.56%.
The Wantz preperitoneal technique
This
is a variation of the Stoppa technique.
Wantz uses a mesh
corresponding to 1
cm less than the distance between the midline and the anterior-superior
iliac
spine. The depth of the mesh depends on the patient's body size, usually
between
12 and 14 cm. The mesh is inserted (in local anaesthesia) through a
transversal
lateral incision. The transversalis fascia is incised
longitudinally, near
the border of the rectus muscle. A large mesh is introduced into the
preperitoneum and sutured to the wall where more accessible, but at
deeper
seated level, the mesh is positioned between peritoneum and the wall,
without
sutures. The mesh may be fenestrated to permit the passage of the
testicular
vessels or may be positioned above them, once they have been isolated
from the
peritoneum for a considerable distance.
The Nyhus technique
Thanks
to Nyhus, the
preperitoneal approach was relaunched in 1959. He proposes a
suprainguinal
approach and suture of the hernial defect from within. In hernia
recurrences, he
uses a mesh to reinforce the suture of the hernial defect. He uses a cm 6
x 14
rectangle in polypropylene. He fixes it, with unabsorbable stitches, to
the
Cooper ligament and to the posterior suture of the hernia defect. He
positions
it and fixes it with U-shaped stitches, behind the operating wound, to
protect
it (Fig. 5-10). 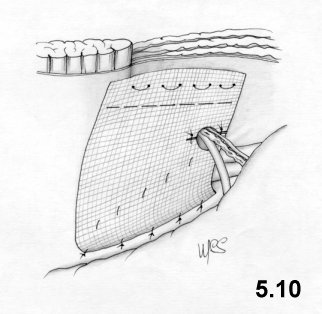
Laparoscopic hernioplasty
E.
Nicolo'
The transabdominal, preperitoneal and completely
extraperitoneal
approaches have already been amply illustrated in chapter 2. The reader
should
therefore refer back to what has already been said for data regarding
the
initial phases of this technique.
The transabdominal
preperitoneal
approach
While the peritoneum is still intact, using hand
pressure on the outside of the abdominal wall, the pubic spine
corresponding to
the midline is identified.
The first structure identified is
the
umbilicus-lateral bladder ligament (the medial border for the dissection
of the
peritoneum).
This ligament may be divided using clips to
obtain a
better vision of the medial portion of the inguinal region. The bladder
should
also be identified so as to avoid damage to it.
Moving down
along the
umbilicus-lateral bladder ligament, we find the deferent canal, which
stemming
from the pelvis follows a medial-lateral path in the direction of the
deep
inguinal ring.
At this level, the deferent canal joins the
internal
spermatic vessels, which follow a lateral-medial path, and form an
upturned V
shape. The highest oint of this V corresponds to the deep inguinal ring
and is
directed upwards as if pointing to the inferior epigastric vessels.
The inferior epigastric vessels are not always easily
identifiable,
especially in obese patients, even when the peritoneum is intact.
The
parietal peritoneum is cut as high as possible (Fig.
5.11) 2-3 cm from the lateral border of the
deep
inguinal ring medially to the
umbilicus-lateral bladder
ligament.
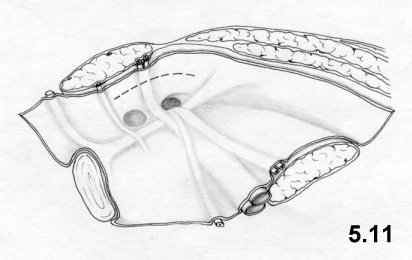
![]()
First, the upper peritoneal flap is dissected smoothly. The lower
peritoneal flap is treated in the same way up to iliac vessel level.
The lower epigastric vessels if not identifiable while the
peritoneum is
intact, they will be when it is opened (Fig.
5.12). 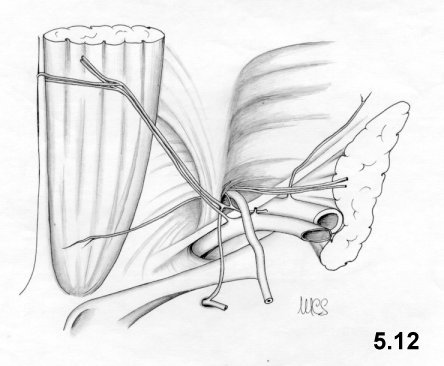
![]()
During the
preparation of the
flaps, particular attention should be paid to the peritoneal vessels.
The
aponeurosis of the transversus muscle is identified above the deep
inguinal
ring. It is then followed medially to its insertion with the Cooper
ligament,
close to the pubic spine.
The iliopubic tract (or Thompson
ligament)
is identified at the lower edge of the deep inguinal ring. It lies
parallel to
the inguinal ligament, is situated closer to the surface and is not
laparoscopically visible.
Following the iliopubic tract,
medially,
the Cooper ligament is then identified. The circumflexa ilii profunda
artery is
easily identified, because it is parallel to the iliopubic tract.
In
indirect hernia, the sac must be carefully isolated from the spermatic
cord and
introflexed. When the sac is too large, it may be resected, as occurs in
the
presence of an adipocele.
In femoral hernia, the Cooper and
Thompson
lacunar ligaments are exposed. The deferent canal and internal spermatic
vessels
are isolated smoothly creating a gap between these elements and the
iliac
vessels.
A Prolene mesh of 7.5 x 12 cm is cut, as illustrated
in Figure 5.13 (a cut
parallel to the
longer side and about 2/3 its length is performed at about 1/3 from
the
bottom of the shorter side).
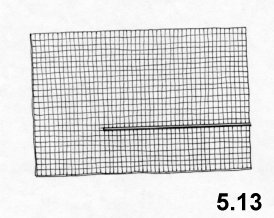
The large flap is used to cover the inguinal ring.
The mesh is fixed, using clips, first onto the Cooper ligament, then onto the abdominal wall to the right and the left of the epigastric vessels. Finally, both borders of the mesh are joined and fixed by clips above the iliopubic tract (Fig. 5.14).
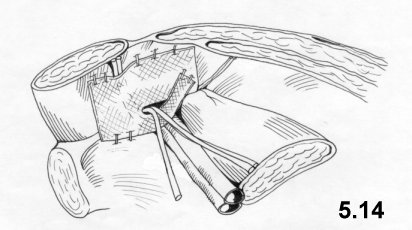
Clips or continuous stitching suture both peritoneal borders. The trocars are moved under direct surveillance and the pneumoperitoneum is reduced. The skin incisions are closed by intradermic suture.
In cases of inguinal-femoral hernia in women, the mesh is not cut as indicated above, but positioned whole and fixed at the same levels, above the round ligament.
The preperitoneal approach
Once the preperitoneal region has been reached, as described in chapter 2, the same situation as in the transperitoneal approach presents itself, once the peritoneum has been opened.
The operating step is similar to that described in the preperitoneal transabdominal approach. The only difference is that no repair of the peritoneum occurs.
References
AJABNOOR M.A., MOKHTAR A.M., RAFEE A.A., TAHA A.M.: Defective collagen metabolism in Saudi patients with hernia. Ann. Clin. Biochem. 29 (4): 430-436, 1992.
AMID P.K., SHULMAN A.G., LICHTENSTEIN I.L.: Critical scrutiny of the open "tension-free" hernioplasty. Am. J. Surg. 165: 369-371, 1993.
AMID P.K., SHULMAN A.G., LICHTENSTEIN I.L.: Femoral hernia resulting from inguinal herniorrhaphy: the "plug" repair. Cont. Surg. 39: 19-24, 1991.
ARNAUD J.P., ELOY R., ADLOFF M., GRENIER J.F.: Critical evaluation of prosthetic materials in repair of abdominal wall hernias. New criteria of tolerance and resistance. Am. J. Surg. 133: 338-345, 1977.
ARNAUD J.P., ELOY R., WEILLBOUSSON M., GRENIER J.F., ADLOFF M.: Résistance et tolérance biologique de 6 prothèses utilisées dans la réparation de la paroi abdominale. J. Chir. 113: 85, 1982.
BARNES J.P.: Inguinal hernia repair with routine use of Marlex mesh. Surg. Gynecol. Obstet. 165: 33-37, 1987.
CAPOZZI J.A., BERKENFIELD J.A., CHEATY J.K.: Repair of inguinal hernia in the adult with Prolene mesh. Surg. Gynecol. Obstet. 167: 124-128, 1988.
CHEATLE G.L.: An operation for the radical cure of inguinal and femoral hernias. Br. Med. J. 2: 68, 1920.
CONDON R.E.: The anatomy of the inguinal region. In: NYHUS L.M., HARKINS H.N. (eds.): Hernia. ed 2., J.B. Lippincott, Philadelphia, 1964, p. 14.
CORBITT J.D., Laparoscopic herniorrhaphy. Surg Laparosc Endosc 1991; 1:23-25.
FILIPI C.J., FITZGIBBONS R.J., SALERNO G.M., HART R.O.: Laparoscopic herniorrhaphy. In: Laparoscopy for the General Surgeon. Surg. Clin. North Am., 1992.
FITZGIBBONS R.J., SALERNO G.M., FILIPI C.J., HUNTER W.J., A laparoscopic intraperitoneal only mesh technique for the repair of an indirect inguinal hernia. Ann Surg 1994; 219(2):144-156.
FITZGIBBONS R.J., Laparoscopic inguinal hernia repair. Paper presented at New Frontiers in Endoscopy Nationwide Satellite Teleconference. 1991 (May 15).
FRUCHAUD H.: Anatomie chirurgicale des hernies de l'aine. Doin, Paris, 1957.
GATT M.T., CHEVREL J.P.: The treatment of neuralgia following inguinal herniorrhaphy: a report of 47 cases. Postgrad. Gen. Surg. 4 (2): 142-147, 1992.
GILBERT A.I.: An anatomical and functional classification for the diagnosis and treatment of inguinal hernia. Am. J. Surg. 157:331-333, 1989
GILBERT A.I.: Inguinal hernia repair. Biomaterials and sutureless repair. Perspectives in Gen. Surg. Vol. 2, 1: 113-129, 1991.
GILBERT A.I.: Sutureless repair of inguinal hernia. Am. J. Surg. 163:331-335, 1992
HALSTED W. S.: The radical cure of inguinal hernia in the male. Bull. of the Johns Hopkins Hospital IV, 29: 17, 1893.
HARRISON P.W.: Inguinal hernia. A study of the principles involved in surgical treatment. Arch. Surg. 4: 680, 1922.
HAWASLI A.: Laparoscopic inguinal herniorrhaphy: Classification and 1 year experience. J. Laparoendoscopic Surgery, 1992.
HENRY A.K.: Operation for femoral hernia by a midline extra peritoneal approach. Lancet 19: 531-533, 1936.
KAVIC M.S., Laparoscopic hernia repair. Surg. Endosc 1993; 7:163-167
KAVIC MS.: Laparoscopic hernia repair. Harwood Academic Publishers Amsterdam 1997
LICHTENSTEIN I.L., SHORE J.M.: Simplified repair of femoral and recurrent inguinal hernias by a "plug" technique. Am. J. Surg. 128:439-444, 1974
LICHTENSTEIN I.L., SHULMAN A.G., AMID P.K., MONTLLOR M.: Cause and prevention of post-herniorrhaphy neuralgia: a proposed protocol for treatment. Am. J. Surg. 155: 786-790, 1988.
LICHTENSTEIN I.L., SHULMAN A.G., AMID P.K.I. et al: The tension-free hernioplasty. Am. J. Surg. 157:188-193, 1989
LICHTENSTEIN I.L., SHULMAN A.G.: Ambulatory outpatient hernia surgery, including a new concept, introducing tension-free repair. Int. Surg. 71:1-7, 1986
MAHORNER H., GOSS G.M.: Herniation following destruction of Poupart's and Cooper's ligaments: a method of repair. Ann. Surg. 155: 741-747, 1962.
McKERNAN J.B., LAWS H.L. Laparoscopic repair of inguinal hernias using a totally extraperitoneal prosthetic approach. Surg. Endosc. 1993; 7:26-28.
NOLEN M., MELICHAR R., JENNINGS W.C., McGEE M.C.: Use of a Marlex fan in the repair of direct and indirect Hernias by laparoscopy. Laparoendoscopic Surg., 1992.
NYHUS L.M. et al.: The preperitoneal approach and prosthetic buttress repair for recurrent hernia: the evolution of a technique. Ann. Surg. 208: 733-737, 1988.
NYHUS L.M., CONDON R.E., HARKINS: Clinical experiences with preperitoneal hernia repair for all types of hernias of the groin. Am. J. Surg. 100: 234, 1960.
NYHUS L.M.: Inguinal hernia. Curr. Prob. Surg. XXVIII-6: 406-450, 1991.
PEACOCK J.R. E.E.: Wound repair. 3rd Ed, WB Saunders Co., Philadelphia, 1984, pp. 336-337.
PEACOCK E.E.: Here we are again: behind again! Am. J. Surg. 157: 187, 1989.
QUILICI P.J.: New Developments in Laparoscopy. CT: USS P. Press, Norwalk, 1992.
READ R.C.: A review: the role of protease-antiprotease imbalance in the pathogenesis of herniation and abdominal aortic aneurysm in certain smokers. Post. Gen. Surg. 4 (2): 161-165, 1992.
RIVES J. FLAMENT J.B., DELATTRE J. F., PALOT J. P: Traitement des hernies de l'aine à l'aide de prothèses mises en place par voie inguinale directe. Travaux du GREPA, Ed. Bruneau 5: 18-24, 1983.
RIVES J., FLAMENT J.B., DELATTRE J.F., PALOT J.P.: La chirurgie moderne des hernies de l'aine. Cah. Med. 7: 1205-1218, 1982.
RIVES J., FORTESA L., DROUARD F., HIBON J., FLAMENT J.B.: La voie d'abord abdominale sous-péritonéale dans le traitement des hernies de l'aine. Ann. Chir. 32: 245-255, 1978.
RIVES J., LARDENNOIS B., FLAMENT J.B., CONVERS G.: La pièce en tulle de Dacron, traitement de choix des hernies de l'aine de l'adulte, à propos de 183 cas. Chirurgie 99: 564-575, 1973.
RIVES J., LARDENNOIS B., FLAMENT J.B., HIBON J.: Utilisation d'une étoffe de Dacron dans le traitement des hernies de l'aine. Acta Chir. Bel. 70: 284-286, 1971.
RIVES J., LARDENNOIS B., HIBON J.: Traitement moderne des hernies de l'aine et de leurs récidives. Encyclopédie Med. Chir. Techniques Chirurgicales 1 (40 110): 1-12, 1973.
RIVES J., NICAISE H., LARDENNOIS B.: A propos du traitement chirurgical des hernies de l'aine. Orientation nouvelle et perspectives thérapeutiques. Ann. Med. Reims 2: 193-200, 1965.
RIVES J., NICAISE H.: A propos des hernies de l'aine et de leurs récidives. Sem. Hòp. 31: 1932-1934, 1966.
RIVES J., STOPPA R., FORTESA L., NICAISE H.: Les pièces en tulle de Dacron et leur place dans la chirurgie des hernies de l'aine. Ann. Chir. 22: 159-171, 1968.
RIVES J.: Surgical treatment of the inguinal hernia with Dacron patch. Int. Chir. 47: 17) 360-361, 1967.
ROBBINS A.W., RUTKOW I.M: The Mesh-plug hernioplasty. Surg Clin NA 73: 501-512,1993
SCHULTZ L., GRABER J. PIETRAFITTA J., HICKOK D.: Laser laparoscopic herniorrhaphy: A clinical trial, preliminary results. J. Laparoendoscopic Surg. 1: 41-45, 1990.
SHULMAN A.G., AMID P.K., LICHTENSTEIN I.L.: Prosthetic mesh plug repair of femoral and recurrent inguinal hernias: the American experience. Ann. Royal Coll. Surg. Engl. 74: 97-99, 1992.
SHULMAN A.G., AMID P.K., LICHTENSTEIN I.L.: The safety of mesh repair for primary inguinal hernias: results of 3,019 operations from five diverse surgical sources. Am. Surg. 58: 255-257, 1992.
STARLING J.R., HARMS B.A.: Diagnosis and treatment of genitofemoral and ilioinguinal neuralgia. World J Surg 13:586-591, 1989
STOPPA R., PETIT J., ABOURACHID: Procédé original de plastie des hernies de l'aine. L'interposition sans fixation d'une prothèse en tulle de Dacron par voie médiane prépéritonéale. Chirurgie 99: 119, 1973.
STOPPA R.E., RIVES J.L., WARLAUMONT C.R. et al: The use of Dacron in the repair of hernias of the groin. Surg. Clinic. North Am. 64: 269, 1984.
STOPPA R.E., WARLAUMONT C.R.: The midline preperitoneal approach and the prosthetic repair of groin hernia. In: NYHUS L.M., BAKER R.J. (eds.): Mastery of Surgery. 2nd ed., Little Brown Ed., Boston, 1992.
STOPPA R.E., WARLAUMONT C.R.: The preperitoneal approach and prosthetic repair of groin hernia. In: NYHUS L.M., CONDON R.E. (eds.): Hernia. 3d ed, J.B. Lippincott, Philadelphia e Toronto, 1 vol., 1989.
USHER F.C., ALLEN J.E., CROSTHWAIT R.W., COGAN J.E.: Polypropylene monofilament. A new, biologically inert suture for closing contaminated wounds. JAMA 179: 780, 1962.
WAGH P.V., LEVERICH A.P., SUN C.N., WHITE H.J., READ R.C.: Direct inguinal herniation in men: a disease of collagen. J. Surg. Res. 17: 425, 1974.
WANTZ G.E.: Atlas of Hernia Surgery. 1 vol. Raven Press Ed., New York, 1991.
WANTZ G.E.: Giant reinforcement of the visceral sac. Surg. Gynceol. Obstet. 169: 408, 1989.
WILLIS I.H., SENDZISCHEW H.: Laparoscopic pre-peritoneal prosthetic inguinal herniorrhaphy. Laparoendoscopic Surg., 1992.
PART TWO
PHYSIOLOGICAL HERNIOPLASTY
6 A RE-EXAMINATION OF THE INGUINAL REGION FROM AN ANATOMICAL AND FUNCTIONAL POINT OF VIEW
The inguinal region has been studied thoroughly. However, I think that some important anatomical and physiological aspects should be given greater consideration. Therefore, I should like to highlight these aspects, in order to discuss the theoretical premises of the physiological hernioplastic method.
The anatomical and functional aspects of the anterior abdominal wall
The anterior abdominal wall is composed of muscles, fascias and
aponeuroses.
The fascias cover the muscles. The aponeuroses are flat tendons,
anatomical
continuations of the corresponding muscles characterized by the collagen
fibers
aligned with those of the muscles.
The aponeuroses are considerably
resistant to traction in the direction of the fibers. When traction
occurs in a
transversal direction, the aponeuroses are not so resistant but are
elastic.
The muscles and fascias are located in such a way as to form a synergic
system: at rest, the fascias contain and protect the muscles while,
under
strain, the muscles protect the fascias, because as they contract they
become
very rigid.
The posterior wall of the inguinal canal and the linea
alba are
the only areas not sustained by muscles in the anterior abdominal wall.
In
normal conditions these are very narrow. We use the expression
passive area to indicate an area of the abdominal wall
consisting
only of fascia, but no muscle, because it does not react actively to the
increase in internal pressure caused by the prelum abdominale.
When the abdominal muscles contract:
- the muscles hardens
and shortens
- the diameters of the abdominal cavity are reduced
- the
fascial zones,
protected by the muscles, and not subject to stress, relax
- the
aponeuroses
are placed under tension in the direction of their fibers
- the
endoabdominal pressure increases considerably
- the fascial zones
which are
not protected by muscles (passive areas) receive a solicitation
from an
endoabdominal pressure proportionate to the dimension and to the bend
radius of
their surfaces.
To clarify this last statement, it is necessary to consider the
effects of
the endoabdominal pressure on a passive area according to Laplace's
law.
Laplace's law applies to elastic cylindrical or spherical
containers, which undergo an internal pressure greater than the external
one:
the relationship between the transmural pressure (P), the tension of the
wall in
a point (T) and the bend radius of the wall in that point " is T= P x R
in a
cylindrical container, T= P x R/2 in a spherical container".
More
simply, the tension caused by a constant internal pressure upon each
point of
the wall, in an elastic cylindrical or spherical container, is
proportional to
the bend radius at that point. For example, when a cylindrical
balloon is
inflated, the proximal tract inflates first and this increases the
length of its
radius.
As the air enters, the proximal tract inflates while the
distal
tract, which has a shorter radius, does not and is less stressed even
though it
is subject to the same pressure (Fig. 6.1). It is surprising to observe how soft the wall of the
undilated
tract is and how little resistance it opposes, in contrast with the
obvious
tension and hardness of the dilated tract.
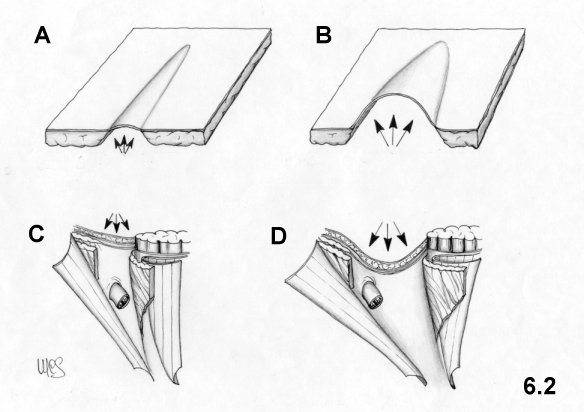
The structural aspects of the main anatomic layers of the inguinal region in normal conditions and in hernia patients
In males, the inguinal region represents a critical area due to a number of peculiar characteristics:
- the inguinal canal is weak because the spermatic cord which is soft, mobile and opposes no resistance to stress, passes through it
- there is more presence of fascial and aponeurotic tissue and little muscle presence
- the deep and superficial rings represent two weak points.
The posterior layer of the inguinal region consists of the transversalis fascia and of the transversus muscle with its aponeurosis. The deep ring is located at this level.
The transversus muscle is not so well represented and borders on the supero-external side of the deep ring; its aponeurosis borders on the inferior-medial side of the deep ring.
The passive area, corresponding to the posterior wall of the inguinal canal, is formed by the transversalis fascia and by the iliopubic tract. This area is not very strong but is very narrow. Medially the aponeurotic arch of the transversus muscle, adheres to the transversalis fascia, covered by the internal oblique muscle and is characterized by curved and rather thin fibers.
Often in hernia patients, the posterior wall of the inguinal canal is usually wide and thin. In the indirect hernia the deep ring is quite wide, surrounded by weak tissues with adipocele on the lateral side. The transversalis fascia is very thin and often unrecognizable from the external side of the deep ring.
The intermediate layer
The intermediate layer is constituted by the spermatic cord and by the internal oblique muscle. The spermatic cord is soft and therefore alters the synergy existing between the anatomical planes. The internal oblique muscle with its free margin surrounds the funiculus at the exit of the deep ring; then, it continues along the funiculus as far as the pubis. In this way, it forms the whole medial margin of the inguinal canal. The distance between the internal oblique muscle and the inguinal ligament is minimal.
In hernia patients (except for some congenital hernias affecting young people), the lower margin of the internal oblique muscle seldom reaches the pubis. Its inferior fibers reach the rectus sheath at a variable distance from the pubis, even 3-4 cm. Therefore, a passive area, the inguinal triangle (Fig. 6.3) is formed. This triangle shouldn't be confused with the triangle of Hesselbach. Bordering this inguinal triangle are the internal oblique muscle, rectus sheath and the inguinal ligament. Very often the internal oblique muscle is hypotrophic.
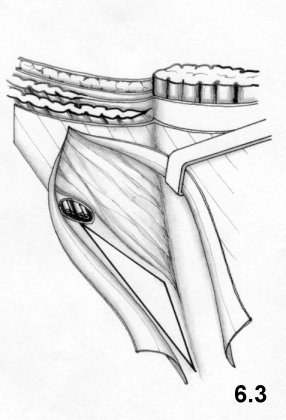
The superficial layer is represented by the external oblique aponeurosis which is characterized by its oblique-oriented fibers.
In hernia patients, the external oblique aponeurosis may be fiberdeficient or hypotrophic at the level of the superficial ring, but usually it does not present large lesions, because the fibers are longitudinally resistant and transversally elastic, unless the hernia is very vast, does not lead to relevant damage.
The normal defense mechanisms of the inguinal region (sling, sphincter and shutter mechanisms)
As an effect of the prelum abdominale, the aponeuroses of the
inguinal canal
are subjected to two solicitations: one is the contraction of the
muscles and
the other is an increase in endoabdominal pressure. The former acts only
in the
direction of the fibers, while the latter is also transversally. The
transversalis fascia is not stressed by the muscular traction, but only
by the
endoabdominal pressure. As stated, the aponeuroses do not resist much to
traction along the transversal axis of the fibers. Therefore, the
solicitation, which finds the inguinal canal in a position of greater
vulnerability is that provoked by endoabdominal pressure acting in all
directions.
The normal defense mechanisms of the inguinal canal
protect
from the action of the prelum abdominale the two critical spots: the
deep ring,
and the posterior wall of the inguinal canal represented only by the
transversalis fascia and by the iliopubic tract.
Protection of
the deep
ring
The contraction of the transversus muscle causes three
effects:
- it narrows the ring,
- it moves the ring in a lateral-cranial
direction.
- it pulls in a lateral-cranial direction the superior
crus of
the transversalis fascia's sling. As a consequence, the exceeding
transversalis
fascia, surrounding the deep ring, closes on the deep ring itself like
an eyelid
(sling effect).
At the same time, the internal oblique
muscle,
contracts, stiffens and lowers itself in front of the deep ring. This
combined
action of the two muscles is called sphincter effect.
Protection
of the posterior wall of the inguinal canal
The simultaneous
contraction
of the transversus and the internal oblique muscles creates the shutter
mechanism: the aponeurotic arch of the transversus stiffens,
straightens and
draws close to the iliopubic tract. The same occurs for the arch formed
by the
internal oblique muscle.
In this way, the posterior wall of the
inguinal
canal is at its most narrow state and is barely stressed by the
endoabdominal
pressure, according to Laplace's law.
The functional aspects of the inguinal region in hernia patients
In hernia patients, these defense mechanisms are seriously
altered.
The
sling and sphincter mechanisms can be preserved in direct hernia, but
they can
be severely altered in indirect hernia.
The shutter mechanism cannot
happen
at the inguinal triangle, even if the internal oblique muscle
contracts a
lot. This passive area can narrow, but it cannot be covered by the
muscle.
When the internal oblique muscle is atrophic, or so weak, as to fail
by
contracting to act as a defensive barrier, it behaves as a passive
area.
In this case, the fibers of the aponeurotic arch of the transversus too
will
undergo solicitations transversally and will easily dissociate one from
another.
The posterior wall of the inguinal canal has a function we have
called
sealing: a gap in the posterior level will certainly determine
the exit
of a hernia, which cannot be stopped by the upper layers.
We have
many
doubts about the emphasis given to the resistance of the transversalis
fascia.
It can be very resistant to solicitations only when the posterior wall
of the
inguinal canal is narrow and when the protective action of the upper
layers is
efficacious.
There is no doubt that the inguinal region wall is a
synergic
system where each of its levels has a specific role. In any case, I have
noticed
up to now, that the posterior wall has been overestimated, while the
other two
anatomic levels, even if indirectly involved in the formation and
development of
a hernia, have been underestimated.
The other two anatomic layers
are: the
internal oblique muscle, which is certainly the most important active
defender
of the inguinal canal and the external oblique aponeurosis, which has a
very
important role in supporting the layers beneath.
I would like to
underline
the importance of the inguinal triangle as a weak passive area.
It
is often wide and unprotected by the shutter mechanism. Concluding, I
would like
to point out that narrowing a passive area means reinforcing it.
According to what has been pointed out, the purposes of a
"functional"
hernial therapy is:
- repair of the lacking sealing function
of the
posterior layer
- repair of the lacking sphincter effect at the
level
of the deep ring
- repair of the lacking shutter mechanism.
Repair of the seal function of the posterior layer
It is
very
important to separate the elements of the funiculus from the proximal
tract of
the cremaster, in order to eliminate even small adipoceles, because they
stick
to the cremaster and easily lead to recurrences. A perfect calibration
of the
deep ring around the cord elements is no less important. Anyway, one
must
consider that even after a good repair has been performed, the poor
quality of
the tissues surrounding the ring, make the risk of laceration greater.
Repair of the posterior wall of the inguinal canal is fundamental in
direct
hernia. In this case, sometimes the stitches passing through an entire
wall, may
cut the tissues and open new hernial defects.
Repair of the
sphincter
effect at deep ring level
The action (narrowing and rising the
deep ring
so that it be protected by the internal oblique muscle) of the
transversus
muscle may be repaired as long as the deep ring remains on the same
anatomical
level, free and independent from the levels above.
Repair
of the
shutter mechanism
In order to repair the shutter mechanism, the
inguinal
canal must be narrowed and must border on the entire inferior-lateral
side of the internal oblique muscle. This means that if the lower
lateral
margin of the internal oblique muscle is short and it inserts high on
the rectus
sheath, the inguinal canal has to be short and end at the same height of
the
rectus sheath.
The inguinal triangle has, therefore, to be
excluded from
the inguinal canal. Like every other passive area, it has to be
reinforced
and narrowed, compatibly with a moderate tension of the sutures.
These
premises are necessary to explain the technical choices of the
physiological
hernioplasty. Its purpose is to repair the defense mechanisms of the
inguinal
region respecting the principles of physics and biology.
The
myopectineal orifice
According to Fruchaud, the myopectineal orifice is bordered,
medially by the
rectus sheath, superiorly by the transversus and internal oblique
muscles,
laterally by the iliopsoas muscle, inferiorly by the pubis covered by
the Cooper
ligament and by the pectineus muscle with its fascia. Basically, this
perspective believes that the two weak areas of the inguinal and crural
regions
are essentially one. The iliopubic tract and the inguinal ligament,
which cross
and divide the myopectineal orifice, are considered supporting elements
of minor
importance.
This position has been supported by those who advocate
suturing
or better still insertion of meshes into the entire weak area
corresponding to
the myopectineal orifice. Reality has belied this perspective: crural
hernia is
far less frequent than inguinal hernia. Evidently, the iliopubic tract
and the
inguinal ligament have very important roles as "support beams", also
because
they occur in an angular area, so that, according to Laplace's law, the
stress
is reduced due to the existence of a short bend radius.
I believe it
is
better to limit the surgical trauma. Too often supporters are
overenthusiastic about the "myopectineal orifice" and this leads to the
practice of ample dissections and applications of meshes anchored to the
Cooper
ligament without any specific indication.
7 WHY A NEW METHOD?
Traditional methods tend to repair normal anatomy, without respecting
the
physiology of the area. It is well-known that sutures under tension or
which go
through the whole wall can open new hernial defects, create ischemia and
impede
perfect healing. Recurrences exceed widely the 10% in patients not
treated in
specific hernia units.
Meshes substitute and reinforce tissues,
avoid suture
tension, but they do not eliminate passive areas. Physiology of the
inguinal
region is hardly ever respected even in prosthetic surgery. Recurrences
are
definitely reduced with meshes compared to traditional methods. But even
if mesh
technique is expanding today, direct suture still remains the most
common.
Today, some prosthetic methods have a recurrence incidence of close
to 0%.
Is there any possibility for further progress? Is there any necessity
for a
"post mesh"?
I usually use mesh and think, that in many cases
they
are useful and in some absolutely necessary. But, I do not believe they
should
be used indiscriminately. Even if their side effects are rare, when they
arise
they can be serious. Even if the main objective, that
is recurrence, is
avoided; patients, as they often refer, may have an unpleasant
subjective
perception of the prosthesis after recovery. I would also like to
remark, even
if a foreign body is biocompatible, it is always artificial and
unnatural.
That is why I propose a technique, which is not a return to the
past, but
belongs to the "post mesh" era. I think the time has come to give its
due weight
to physiology, so often emphasized yet so often neglected. I also
believe
that the biology of tissue should be more respected. Biology is
not
respected by substituting tissues with foreign inert bodies, even if
sutures are
without tension.
Today, the repair of the defense mechanisms and
respect of
the biology of tissues can be harmonized.
8 PHYSIOLOGICAL HERNIOPLASTY
To understand the principles and objectives of my inguinal hernia
surgical
repair technique it is sufficient to refer back to chapter 6.
My
technique
was devised in 1988 for two purposes:
- restoration of the defense
mechanisms of the inguinal region through repair which adapts the
anatomy to
functional necessity.
- respect of the biology of sutured tissues.
These
two objectives always considered of major importance, have never been
simultaneously achieved by traditional or modern methods.
The
technique
Common
preliminary
steps
- The best skin incision is transversal, because it heals better.
- The cribriform fascia is cut slightly below the inguinal ligament to free the external oblique aponeurosis on the lateral side and to check for the presence of a subclinical crural hernia.
- The external oblique aponeurosis is incisedfg along the fibers as far as the opening of the superficial ring.
- The medial flap of the external oblique aponeurosis is dissociated from the layer below. The separation should be quite wide in order to uncover the rectus sheath as much as possible.
- The funiculus is isolated at the level of the pubic spine and pulled laterally.
- The inferior border of the internal oblique muscle is separated from the medial fibers of the cremaster (Fig. 8.1).
- The cleavage between internal oblique muscle and aponeurotic arch of the transversus muscle is detected. The internal oblique muscle is retracted medially and the layer constituted by the aponeurosis of the transversus and transversalis fascia is exposed medially to the deep ring, for a wide tract.
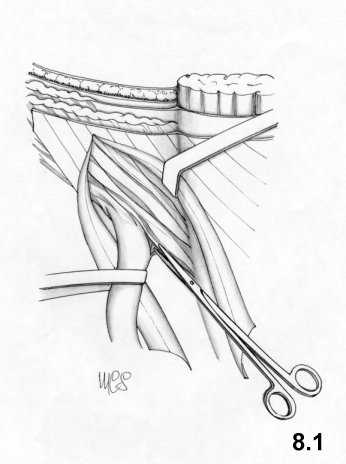
The treatment of the deep layers is different in indirect hernia with a medium-small defect and in direct or indirect hernia with a large defect.
Indirect hernia with a medium-small defect.
- An incision on the funiculus involving the proximal tract of the internal spermatic fascia as far as the deep ring, is performed.
- The sac, beyond the neck, is isolated. It can be either resected or simply pushed in the preperitoneum. I prefer the second choice, unless the sac is very long and adherent. In this case, I divide it leave the fundus in situ.
- The elements of the funiculus (vessels and deferent) are separated from the proximal tract of the internal spermatic fascia and cremaster and then isolated. The isolation is extended to the level of the deep ring and, for a few centimeters, in the preperitoneal area.
- A two-centimeter incision is performed on the transversalis fascia and aponeurosis of the transversus, starting on the deep ring, in a medial and cranial direction (Fig. 8.2).
- The elements of the funiculus are brought to the medial angle of this incision (Fig. 8.3); then, the first layer of suture is started. With the first passage of the thread, a new easily calibrated deep ring is created (Fig. 8.4). The incision is then sutured until the original ring is completely closed (Fig. 8.5). Keeping the same suture, a second layer is created, but in the opposite direction, to cover the first layer with the cremaster and internal spermatic fascia. (Figs. 8.5, 8.6).
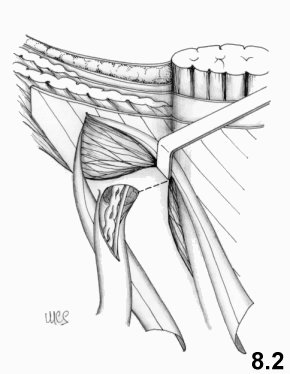
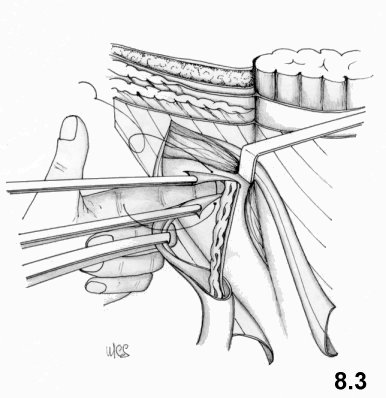
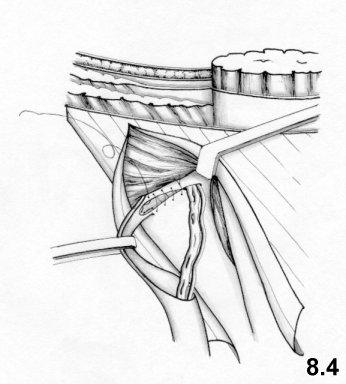
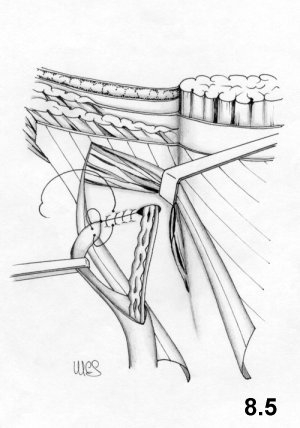
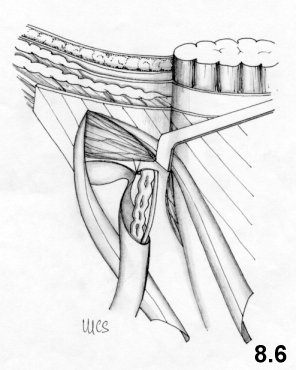
Direct hernia or indirect hernia with a large defect.
My technique consists in partially repairing the posterior wall
of the
inguinal canal so as to transform the large defect into a small-defect
indirect
one. The surgery is then completed following the repair technique for a
small-defect external oblique hernia.
The common preliminary
procedure has
already been described.
Having isolated the funiculus and retracted
the
internal oblique muscle the rest of the operation will follow a precise
pattern
depending on the nature of the hernia.
In indirect hernia, a
medial
incision is performed, which involves the proximal tract of the internal
spermatic fascia and the deep ring. A second incision on the
transversalis
fascia of the deep ring up to the pubic spine is performed.
In
direct
hernia, the transversalis fascia above the hernial sac is resected.
Then, an
incision on the transversalis fascia is extended cranially, up to
the deep
ring.
The sac, both in direct and indirect hernias, is
isolated
beyond the neck, and simply pushed into the preperitoneal cavity. Only
in big
scrotal hernias, the sac is resected leaving the body and fundus in
situ.
- The preperitoneum is detached from the transversalis fascia medially to the hernial defect, beyond the lateral margin of the rectus sheath.
- The posterior wall of the inguinal canal is partially closed through overlapping of the flaps with a continuous suture. The suture joins the iliopubic tract to the internal surface of the transversalis fascia at the level of the lateral margin of the rectus muscle (Fig. 8.7). It starts at the pubic spine and stops at the level of the inferior epigastric vessels. The left-over medial flap of the transversalis fascia is joined, using the same continuous suture, to the iliopubic tract. (Fig. 8.8). The suture does not involve the inguinal ligament and only touches lightly, the deep ring, which is left open.
- Then one proceeds as if dealing with an indirect hernia with a medium-small defect: the internal oblique muscle is retracted and an approximately 2-centimeter incision with a medial direction is performed on the transversalis fascia beginning from the deep ring (Fig. 8.8).
- The elements of the funiculus are separated from the internal spermatic fascia + cremaster and dislocated to the medial angle of this incision. A continuous suture creates a deep neo-ring, unites the borders of the incision and, after the deep primitive ring has been completely closed, on the way back, covers the suture line with the cremaster. (These surgical manoeuvres have been described in details; cf Figs. 8.3, 8.4, 8.5, 8.6).
- In cases in which the posterior wall of the inguinal canal is wide and strong, instead of repairing with overlaying flap, a plication of the transversalis is carried out to narrow the inguinal canal: a continuous suture joins the iliopubic tract to the aponeurotic arch of the transversus, from the deep ring to the pubis.
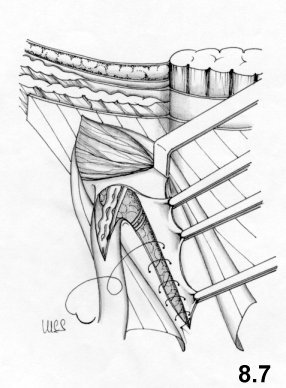
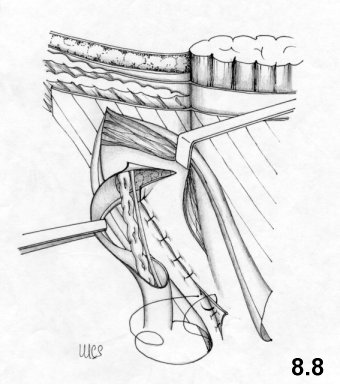
Treatment of the superficial layer
The repair of the superficial layer is the same as in all hernias.
- The external side of the inferior-lateral border of the external oblique aponeurosis is freed completely from every adhesion.
- The point at which the inferior part of the internal oblique muscle reaches the rectus sheath is found. At this level a new superficial ring is created: the suture is performed between the margin of the inferior-lateral flap of the external oblique aponeurosis and the rectus sheath, along a line parallel and 1 cm medially from the lateral margin of the rectus muscle. The suture runs up to the pubis (Fig. 8.9), while the funiculus is kept laterally to the operation field. Therefore, the suture is behind the funiculus. Usually, we use continuous suture in both directions, so that it is easier to tie the thread.
- The funiculus is relocated in its place, leaning completely on the internal oblique muscle.
- Enough space is left for the exit of the funiculus (Fig.8.10), and a second suture between the rectus muscle sheath and the lateral margin of the external oblique aponeurosis, along the previous line, is performed. The third suture level is, therefore, completed.
- The fourth layer is characterized by the suture which involving the superior-medial flap of the external oblique aponeurosis and the external side of the inferior-lateral aponeurotic flap, so that there is wide overlapping without much tension. This suture level, as the previous one, is antefunicular and proceeds from the neo- superficial ring to the cranial extreme of the incision (Fig. 8.11) and retrofunicular from the superficial neo-orifice to the pubis (Fig. 8.12).
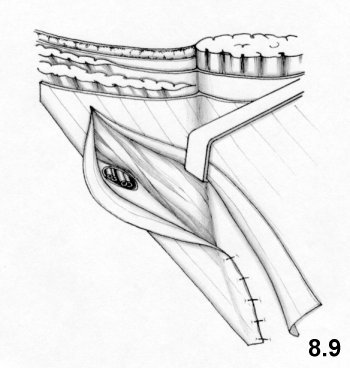
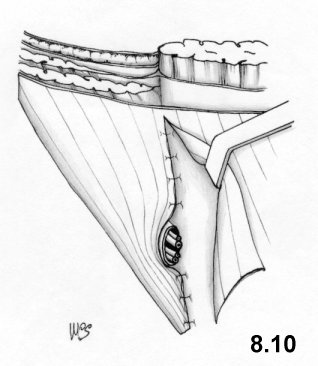
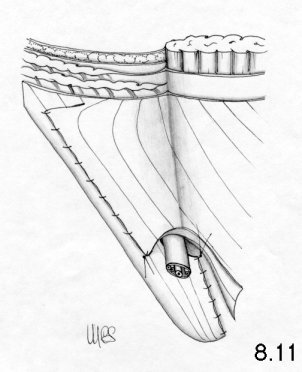
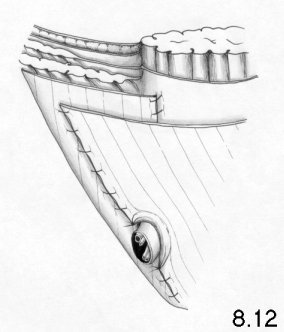
In case of the poor-quality tissue, this technique may include the use of mesh.
The suture materials
We use absorbable sutures
(polyglicolic acid) and nonabsorbable sutures (polypropylene). The
latter
only in cases in which polypropylene mesh are used.
Isolation of the funicular elements from the proximal tract of
the
cremaster
After having opened the internal spermatic fascia, the
isolation of the vessels is very easy. The deferent duct is more
adherent to the
surrounding tissues, but easily recognizable because of its consistency.
Usually
it is possible to see the peritoneal reflection stuck to the elements of
the
funiculus. We dissociate it for a few centimeters to avoid its passing
into the
deep ring which might lead to a hernia recurrence. It is necessary
to
resect any adipoceles, even the small ones, frequently located on the
internal
side of the cremaster.
Exposition of the layer:
transversalis fascia
+ aponeurosis of the transversus muscle
After isolating the
hernial sac,
two Kelly forceps are put on the medial border on the deep inguinal
ring, next
to the preperitoneal fat, and also a light upward traction is performed.
With a
Russian forceps, the adipose tissue below is pinched and pushed inwards.
In this
way, the separation of the layers becomes easy. The transversalis
fascia
can be well detected at deep level. The Kelly forceps are placed on it
with a
light traction. With the Russian forceps itself, held closed and used as
a
spatula, the fibers of the internal oblique muscle are easily separated
from the
level below. Then, they are pulled back medially, in order to uncover
widely the
transversus arch.
Incision of the transversalis fascia and
creation of
new deep ring
As said, two Kelly forceps are placed on the
border of the
transversalis fascia at the level of the deep ring. The caudal forceps
is
removed, passed below the funicular elements and reattached at the same
spot.
The fascia is kept in traction with the forceps and incised with
the
scissors ( Fig. 8.13).
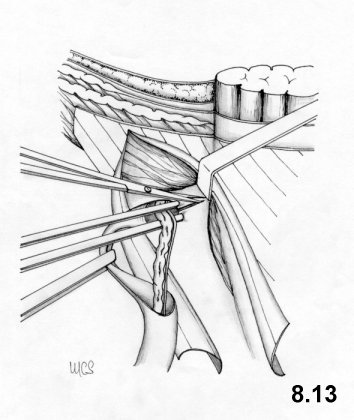
At this point, the traction
applied to the internal oblique muscle should be high to
better expose the
operation field. A two-centimeter medial incision is performed. If
the
internal oblique muscle is poor, the incision is directed more
cranially, so
that the new ring can be covered by the muscle when the retractor is
removed.
The funicular elements are dislocated at the inner angle of the cut and
pulled
medially.
The two Kelly forceps are put close and pulled with the
left hand,
with the palm upwards.
The borders of the incision have to be very
close.
The index finger of the same hand, introduced in the preperitoneal
space, pushes
the funicular elements on the medial border of the incision. In this
position,
the first stitch of the continuous suture is performed (Fig.
8.3), then tied to give the correct
calibration to
the ring.
Closure of the deep ring
The continuous suture
is
brought about from the neo-ring to the deep ring. Once the deep ring has
been
reached, the suture runs along the external hemicircumference. The
inguinal
ligament, which is close to the lateral edge of the deep ring, should
not be
included in the suture. The ring will be easily closed from the traction
of
thread. The same suture, on the way back, joins the free part of the
cremaster
to the suture line below.
Verification of the ring caliber
The
right caliber of the deep neo-ring and of the superficial neo-ring are
verified
introducing in them a curved Kelly forceps. The deep ring has to be
neither too
narrow nor loosen. The new superficial ring has to be rather wide. If it
is too
narrow, it could be widen with a short transversal incision on the
external
oblique aponeurosis.
Meshes are a great progress in hernia surgery and may be useful
in some
cases, in others indispensable. I do not think, however, that meshes
such be
used merely on principle.
In small hernia defects, when the tissue
is weak,
they should be used to reinforce sutures and tissues as
to perform a
"barrier- function», while the defect must always be sutured. Only in
large
defects, the mesh substitutes tissue. The priority is reduction of
passive areas
rather than concern about suture traction. Under strain and in a
standing
position tension on the suture is inevitably provoked. Furthermore,
endoabdominal pressure creates stress in the passive areas, in
proportion to
their size.
In primary hernias, if the tissues are very weak,
inelastic and
lacking in quantity, we use meshes to reinforce the hernioplasty. This
occurs in
5% of primary hernia cases. The mesh is positioned in the preperitoneum
or
alternately in front of the posterior wall of the inguinal canal and of
the deep
ring, once it has been closed. Mesh may also be used for prudential
reasons when
the surgeon is not very familiar with the physiological hernioplasty
technique.
We use mesh on principle in crural hernias and in recurrences. In
this case,
we try to close (successfully in most cases) or, in any case, to narrow
the
hernial defect as far as possible.
In crural and recurrent hernias
meshes
are always used and positioned in the preperitoneum.
The use of preperitoneal meshes in primary hernias
The procedure is identical to that already described for direct
or indirect
hernias up to the step where the sac is isolated. The funicular elements
are
separated and well isolated from the cremaster + internal spermatic
fascia.
A rectangular polypropylene mesh, of about 4 x 8 cm is used.
A
cut of
about 2 cm, is made at 1-2 centimeter from the midpoint, perpendicular
to one of
the longer sides of the mesh. (Fig. 9.1).
In the indirect hernia, the mesh is placed in the preperitoneal area through the incision of the transversalis fascia made to create the neo-deep ring. The mesh is then, fixed to the wall, including it in the suture of the transversalis fascia.
In large hernias or in direct hernias, the mesh is passed into the preperitoneal area through the wide incision performed on the posterior wall of the inguinal canal. During the repair phase, we usually fix the mesh to the wall, including it in the suture. (Fig. 9.3).
In both cases, the operation is performed following the same technique, as if the mesh were not used.
The use of meshes in the prefascial area in primary hernia
In the indirect hernia, when the transversalis fascia is weak or
inelastic
and the closure of the deep ring appears unreliable, a polypropylene
mesh of
about 2 x 6 cm is used on the posterior wall of the inguinal canal.
The
mesh
is positioned after the deep ring is closed. A short cut is made on the
medial
side of the mesh at the height of the neo-deep ring. This cut has the
function
to allow the passing of the funiculus but not to create a ring around it
(Fig. 9.4). 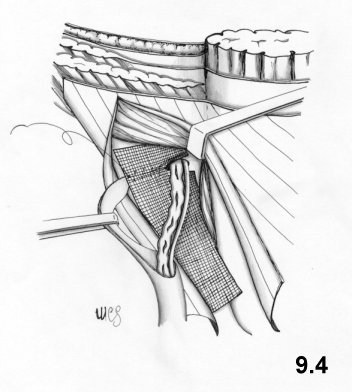
The
thread used for suture, with which the deep
ring has been closed, is posterior to and outside of the mesh. With the
same
thread, backwards, the proximal flap of the cremaster is placed on the
mesh,
including in the suture also the transversalis fascia.
The mesh is
then
spread along the posterior wall of the inguinal canal. The parts of the
mesh
that exceed the medial and cranial sides, are to be positioned under and
not
anterior to the internal oblique muscle.
Other anchoring stitches
are not
necessary. Then, the repair of the overlapping flaps of the external
oblique
aponeurosis is performed.
The use of meshes in large inguinal and crural hernias
Our approach is usually inguinal. We reach the preperitoneum and
the Cooper
ligament, once the posterior wall of the inguinal canal has been opened.
The
polypropylene mesh has a rectangular shape, of about 6-8 cm x 10-12 cm.
The
inferior-lateral angle of the mesh is folded so that length and
inclination of
the edge of the lapover fits the Cooper ligament and so that long side
of the
mesh is parallel to the axis of the groin.
A set of separate suture
stitches
is passed through the Cooper ligament and the mesh, at the level of the
folded
area.
Three- four stitches of monofilament polypropylene begin from
the
pubis and proceed laterally along the Cooper ligament, almost as far as
the
femoral vein. (Fig. 9.5).
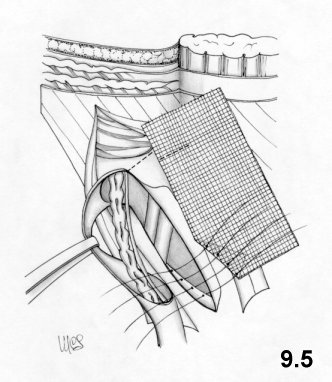
The
suture on the Cooper
ligament is performed more easily if the surgeon moves on the other side
of the
operation field.
The mesh is passed loosely in front of the femoral
vessels
so that the femoral vein does not undergo compression, even when it is
swollen
as in the standing position. A suture unites the mesh to the iliopubic
tract and
to the deep border of the inguinal ligament.
Before the suture has
been
completed, the funicular elements are passed through an eyelet created
on the
mesh.
The mesh is laid in the preperitoneal area.
The further
steps
depend on the conditions encountered. In most primary hernias,
hernioplasty can
be performed as described for direct hernia, anchoring the mesh in the
suture of
the deep layer.
In the recurrence, if necessary, the mesh is sutured
to the
transversalis fascia corresponding to the posterior wall of the rectus
muscle.
The stitches are positioned parallel to the mesh and 1-2 cm away from
medial
border. As far as possible the wall is repaired; in any case the aim is
to
narrow the opening as much as possible and cover the mesh with the upper
layers.
The use of meshes in crural hernias
Large crural hernias are repaired anchoring the mesh to the
Cooper ligament
with the above-mentioned technique.
The anterior approach in the
crural
hernia is simple, but we cannot obtain a good repair directly suturing
the
hernial defect. The Cooper ligament and the transversalis fascia are
very
difficult to reach through a small hernial defect. Then, the lack of
tissues and
their stiffness do not allow the performance of a good suture.
Therefore, the
use of mesh is necessary.
I have developed a technique, called "Locked
-Plug". It is very easy to perform and it permits us to treat crural
hernias
having a small diameter (1-2 cm) with anterior approach. This technique
can be
also used to treat subclinical crural hernias, found during an operation
for
inguinal hernia. In this case, the crural hernia is repaired
independently from
the repair for inguinal hernia.
The approach is anterior. Once the cribriform fascia has been
opened, the
hernial sac is reached, reduced or resected.
It is important to
detach the
sac itself from the hernial defect. A polypropylene square of 4 x 4 cm
is cut. A
thread of monofilament polypropylene is passed through the central point
of the
square and tied. The end of the thread (the part beyond the knot) is
left 10 cm
long and is not cut.
The square-shaped mesh is folded twice along
the
orthogonal lines, crossing the center (Fig. 9.6),
so that
the thread remains in the inside.
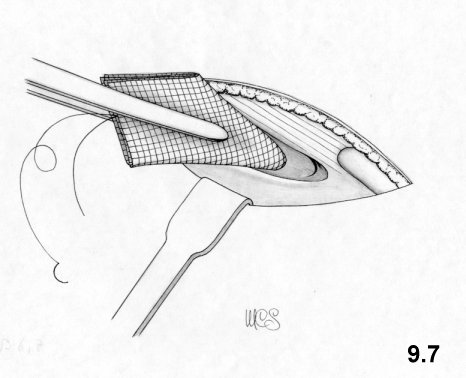
When the forceps is removed, the cone spontaneously tends to expand, helped by the forceps re-introduced into the defect, while a slight traction is applied on the thread.
The same thread is used to suture the borders of the hernial defect. Two stitches are sufficient (Fig. 9.8).
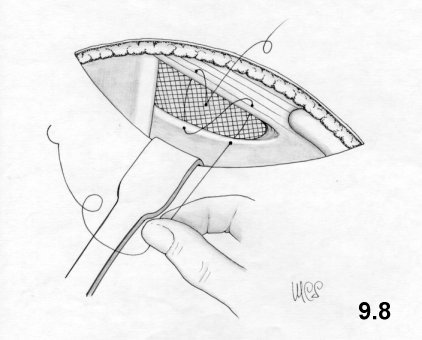
A traction on the tract of the thread linked to the needle narrows the defect and fixes the mesh on it.
The two ends of the thread are, then, tied together.
It is not necessary to include the Cooper ligament and the iliopubic tract in the suture, which cannot be reached through the small defect. It is sufficient to suture the inguinal ligament to the pectineus fascia, which are easily joined.
The purpose of the operation is to narrow the outlet of the canal and plug it with a well-anchored mesh. The holes made by the stitches are protected from the mesh itself, even if they were too wide. Tension of the suture is minimal
The procedure is easy and secure: the suture is limited to only the central area of the hernial defect, at a secure distance from the femoral vein.
The use of meshes in inguinal recurrences
The treatment of recurrence requires great specific surgical
experience, not
only in the repair, but also in the dissection phases and in the
preparation of
the layers and of the spermatic cord.
In the past years, we have
chosen to
minimize dissection and results have proven us to be right. We have
limited
dissection only to the preparation of the sac and the hernial defect,
and in
indirect hernias, to the funiculus. If hernias are multiple and well
separated,
we treat each separately.
This repair always requires the use of
mesh.
We use different repair techniques depending on whether we are
dealing with:
- direct hernia with a small defect
- direct hernia with a medium defect
- indirect hernia
- hernia with a big defect
As in crural hernias, this repair is performed with the Locked-Plug technique.
Direct recurrent hernia with a medium defect (1-3 cm in diameter)
Once the sac has been pushed into the peritoneal cavity and the
defect is
freed from tissue adhesions, a mesh bigger than the hernia defect is
positioned
in the preperitoneum. A continuous suture joins the mesh to the lower
part of
the wall, without passing through its entire depth. The suture then runs
along
the border of the hernial defect, describing a circle having a smaller
diameter
on the mesh. (Fig. 9.9).
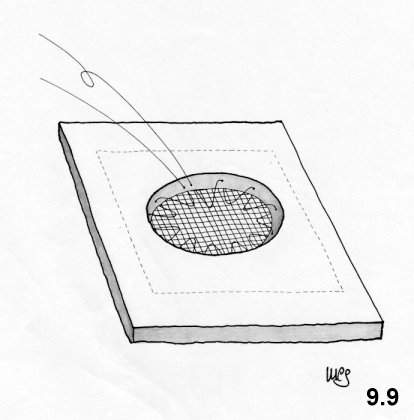
Once
the suture has been completed, the
thread is pulled and tied up. This round suture fixes the mesh and
narrows the
hernial defect while at the same time avoids that the preperitoneal fat
enters
between the mesh and the wall.
Then, another suture joins the edges
of the
hernial defect. This suture is carried out only when there is no
excessive
traction and includes only the superficial level of the wall.
Indirect recurrent hernia with a small - medium defect (1-3 cm in
diameter)
In indirect hernias, once the hernial defect and the
funiculus
have been prepared, a mesh bigger than the hernial defect is
prepared.
The spermatic cord is passed through the mesh through a 2-cm cut and
a
suture is performed, so that a calibrated ring is formed.
The mesh
is
introduced in the preperitoneum through the hernial defect.
If the
defect is
small, it can be sutured directly, calibrated on the spermatic cord and
the mesh
included in the suture.
If the defect is medium size, a circular
suture
between mesh and wall is performed, as described for direct hernia. In
this
case, if there is not much tension, the hernial defect is sutured and
calibrated
on the funiculus.
Hernia recurrence with a large defect (more than 3 cm in
diameter)
Fortunately, hernia recurrences with large defects, associated with
lack and
stiffness of the tissues, are very rare. In this case, it is impossible
to
standardize a procedure: it requires surgical experience and ability. If
the
surgeon is not familiar with the preperitoneal approach, it is better to
anchor
the prosthesis to the Cooper ligament as described above. The mesh must
be
considerably larger than the hernial defect. Therefore, a wide
dissection of the
peritoneum and the ligation of the inferior epigastric vessels are
necessary.
10 CASES AND RESULTS
Francesco Guarnieri
From December 1988 to June 1999, we operated on 2,326 patients
for
inguinal and crural hernia: 1,246 for indirect hernia, 470 for direct
hernia,
335 for double hernia, 226 for recurrent hernia, 49 for femoral hernia.
During
these operations 47 subclinical femoral hernias (2%) have been found.
2,162 were males, 164 were females; the average age was 56 years
(min. 1, max
98).
Local anesthesia was used on 1,710 patients (74 %),
general
anesthesia on 421 patients, spinal anesthesia on 195 patients (8%). The
choice
of the type of anesthesia performed was decided together with the
patient.
Primary direct and indirect hernias
From the onset, physiologic hernioplastic technique has been
applied in 2,051
operations. This technique has undergone a series of marginal technical
variations over time. The most significant change has been the
percentage
variation in use of mesh as a reinforcement in hernioplasty. Up to
January 1998,
when meshes were used, these were positioned in the preperitoneal space
only.
Subsequently prefascial meshes were also employed.
During the first
year,
meshes were used in 49% of all cases. This high percentage was linked to
the
fact that we were not as yet certain of the efficacy of the new
technique. As
the results grew increasingly encouraging, we started to reduce the use
of
meshes drastically. In the second year, we used meshes in 50% of those
cases we
considered as being high risk due to poor tissue quality. Since no
significant
incidence of recurrence occurred, the use of meshes was gradually
reduced to 8%
in 1991. The decision whether to use meshes is usually made in the
operating
theatre, where the quality of the tissues can be evaluated.
Another
important variation is the execution of incisions to relax the rectus
sheath,
which technique we eliminated in February 1996, having verified through
ultrasound and CAT that no significant widening of the rectus muscle
occurred.
Today, we use the relaxing incision of the rectus sheath in those
extremely
rare cases when the rectus muscle is very narrow.
Recurrent hernias belong to a heterogeneous group of cases, where
physiologic
hernioplasty is difficult to perform due to fibrosis. Moreover, in the
last few
years we have reduced dissection to a minimum.
As already stated, in
recurrent hernias we always apply meshes.
The
Locked-Plug
The locked-plug was not only used in crural hernias, but in other kinds of hernias where the defect always presents a diameter of less than 2cm. We have used it in 218 cases: 96 femoral, 57 recurrent, 30 umbilical and epigastric, 23 incisional, and 12 Spigelian hernias. We have treated 47 subclinical crural hernias, detected during surgery for inguinal hernia, with the locked plug.
Since December 1988, patients have been scheduled for follow-up after 7 days, 1 month, 1 year and then annually. Patients who have not followed follow-up programs have been excluded from this survey.
Primary hernias
Here we shall speak mainly about
primary hernias,
the technique has been applied systematically only in this ambit.
Postoperative
complications that arose were: 5% subcutaneous seroma, 1 % temporary
testicular
edema, 0.7 % hematomas, 0.4% wound infection, 0.1% testicular atrophy,
0.7 %
recurrence. It should be noted that no recurrence occurred in those
cases where
meshes were employed.
In the first year, when the femoral ring was
not
systematically explored, 2 cases of crural pseudo-recurrence were found.
After
that no cases of pseudo-recurrence were observed. Subclinical crural
hernia was
found in 2% of patients operated for primary hernia.
Recurrent hernias
Complication incidence rates: 0.8 %
wound
infections, 14 % subcutaneous seroma, 2 % hematomas, 2% testicular
edema, 1.5%
testicular atrophy, 2.6% new recurrence.
LockedPlug
Complications: 6% subcutaneous seroma.
No
recurrence.
11 RATIONALE
My rationale is best understood in the light of Chapter 6. As stated there, this technique aims at restoring the architecture of the inguinal region, so that the defense mechanisms are repaired and the weak areas narrowed and reinforced.
The objective is not to restore the anatomy, but adapt it to functional needs, compensating for tissue weakness, while fully respecting the norms of physics and biology.
The characteristics of the technique are:
- elimination of the deep inguinal ring and creation of a
neo-orifice.
-
narrowing and shortening of the inguinal canal, tailored to fit the
internal
oblique muscle.
- overlap of the external oblique aponeurotic flaps.
-
preservation of the cremaster
The elimination of the
deep and
construction of the new ring
In most
cases the
deep ring is surrounded by weak tissues, unable to resist suture strain.
Its
reconstruction usually proves quite untrustworthy. For this reason
almost all
herniorrhaphy techniques seek to reinforce the deep ring, and anchor it
to the
inguinal ligament, thus, immobilizing and stiffening and
defunctionalizing it.
Besides, the deep ring is not clearly detectable unless the
cremaster is
cut. The tissue weakness, together with defunctionalization and
approximate
suture, are among the chief causes of recurrence.
For this reason,
we have
chosen to create a neo-deep ring, which may be easily calibrated around
the
funicular elements, and positioned in a stronger zone where it can be
protected
by the internal oblique muscle. When the transversus muscle contracts,
the
neo-ring narrows and rises, because it is situated between the fibers of
the
aponeurotic arch. The sphincter effect is thus repaired.
The narrowing and shortening of the inguinal canal
As stated above, the shutter mechanism is
obtained only
when the inguinal canal is narrow and totally surrounded by the internal
oblique
muscle.
In hernia patients, the internal oblique muscle is almost
always
hypotrophic and reaches the rectus sheath "high» relative to the pubic
spine.
The lower zone, unprotected by the muscle, is called the inguinal
triangle (Fig.
6. 3, p. 41). The suture of the external oblique aponeurosis onto the
rectus
sheath is performed so that the inguinal triangle is excluded from the
inguinal
canal and reinforced (Fig. 8.9, p. 50). Consequently, the inguinal canal
becomes
shorter, but functional. The shutter mechanism is thus achieved also
because the
internal oblique muscle is not affected by stitching capable of limiting
its
movement.
Overlapping the external oblique aponeurotic flaps
The external oblique aponeurosis is elastic and
pliable
along the fibers’ transversal axis. By suturing the external aponeurotic
flap to
the rectus sheath, a narrowing of the inguinal canal and of the inguinal
triangle characterized by a moderate degree of suture traction, is
produced. The
medial flap is brought to overlap the lateral one abundantly, producing a
minimum amount of tension. This overlaying distributes the already
moderate
suture traction even more and creates an extensive and compact scar
area, which
prevents the external oblique aponeurosis from fraying. Consequently,
this
aponeurotic layer, as a veritable biological prosthesis, creates new
compact
scar tissue which reinforces the passive areas without tension of the
sutures.
The greatest advantage is obtained at inguinal triangle level, a
passive
area where traditional methods frequently encounter recurrence. In the
inguinal
triangle, the lateral flap of the external oblique aponeurosis, without
the
intrusion of the funiculus, is placed directly on top of the
transversalis
fascia, with which it forms an adherent cicatricial plane. The medial
flap of
the aponeurosis is overlapped once more forming a second scar
plane.
Together, these create a strong wall, certainly no less strong that one
produced
by a mesh.
Preservation of the cremaster
According
to this technique the proximal portion of the cremaster + the internal
spermatic
fascia, free from the funicular elements, are overlapped onto the
suture on
the transversalis fascia between the deep ring and neo-ring. The
cremaster,
besides reinforcing the suture, completely and securely occludes the
deep ring
and blocks any small lacerations which may arise at the passage of the
stitches
on the transversalis fascia below. Further below, the cremaster remains
intact
and protects the testicular vessels from risks of iatrogenic lesions in
case of
an operation for recurrence.
In addition, resection of the cremaster
may
lead to neuralgia of the genital nerve. The cremaster vessels also
guarantee a
collateral vascular bed in cases of testicular vessel lesions.
Discussion
On the basis of
what we have
stated several times above, this technique is highly efficient in repair
of the
functionality of the inguinal canal. Even if the sling mechanism of the
deep
ring is not restored, the results prove that the sealing function of the
posterior wall, at neo-ring level, remains intact. In fact the hernia
recurrences we have encountered after our technique have never occurred
at neo-
deep ring level.
As to narrowing of the posterior inguinal canal
wall, until
February 1996, we supported the idea of the rectus sheath relaxing
incision and
performed it systematically. We held that this relaxing incision served
to
expand the rectus muscle laterally and narrow the inguinal canal. We
were not
interested in reducing the suture tension between the external oblique
aponeurosis and the rectus sheath, which we considered minimal. After
systematic
examinations availing of ultrasound and CAT scans we discovered that
lateral
expansion of the rectus muscle did not in fact occur. We therefore
eliminated
the relaxing incision, which choice produced no changes in either
postoperative
conditions or results. At the same time we paid greater attention to the
question of freeing the lateral flap of the external oblique aponeurosis
from
the lateral fascial tissues to bring it closer to the rectus muscle.
The
technique does not favor the formation of crural hernias. We registered 2
crural
pseudo-recurrences among patients operated, in the first period, when
the fossa
ovalis was not as yet explored. Since we began systematically exploring
the
fossa and treating subclinical concomitant crural hernias (2% of the
cases), no
pseudo recurrence has arisen.
The problem of suture tension and the
poor
healing, (which worries the supporters of the "tension free" school of
taught) need to be addressed.
In our method the
suture lines include only a tiny quantity of tissue and are used
solely to
connect fascias (which do not devascularize); furthermore, they are not
aligned,
nor do they pass through the entire thickness of the wall, thus
receiving
protection from the upper and lower layers which act as a barrier. The
suture
lines on the external oblique aponeurosis are arranged in such a way as
to
re-distribute the already low tension. The overlaping of the flaps
creates ample
contact areas between surfaces developing a solid scar tissue, similar
to that
developed around the meshes. Outcome data accruing to primary hernia
operations
reveal that this technique has a low recurrence rate, the lowest for
techniques
not availing of prosthetic mesh. The recurrences encountered by us were
all
direct smalldefect hernias at either original deep ring level or
immediately below it.
Postoperative complications such as
suppuration,
transitory testicular edema and testicular atrophy comply with most
reports.
It is very difficult for any Author, however self-critical, to point
out the
disadvantages of his/her own method. As similar self-criticism is hardly
credible, I shall here present a number of critical objections I have
received
in a series of questions and answers.
In your large hernia technique, does the
hernia defect
repair not resemble Shouldice’s?
This method and Shouldice’s are
very
different both theoretically and practically; they are similar only in
the
repair of the lower part of the inguinal floor in direct hernias. Above
all,
contrary to Shouldice’s repair, the first line of posterior suture
always
involves the posterior edge of the rectus muscle. Furthermore, the
second line
of stitches along the transversalis fascia does not involve the inguinal
ligament.
In the Shouldice technique, the first suture line includes
the
rectus muscle only along the lower tract; along the upper tract, it
includes the
aponeurotic arch of the transversus because the distance between the
rectus
muscle and iliopubic tract increases considerably as the deep ring is
approached.
Does excessive traction between the rectus muscle and
the
iliopubic tract not occur?The rectus muscle and the transversalis
fascia are
very flexible. Besides, the already moderate amount of suture tension
existing
is reduced by sutures above. Furthermore, it involves the tract between
the
lower epigastric vessels and the pubis where there is less distance
between the
edge of the muscle and the iliopubic tract.
The funiculus forms a
Z-shaped path. Does this not affect the blood supply to the testicle?The
sinuosity of the path of the vessels does not impede the blood flow. In
fact,
the incidence of transitory edema (2%) and testicular atrophy (0.1%) in
males
operated on for primary hernia is low and in keeping with authoritative
reports.
Is the funiculus not too tight in the new inguinal canal,
occupied, as it
is now by the internal oblique muscle?When the muscle tissue is at
rest, it
is soft and compresses the small vessels only slightly, as per Laplace's
law.
The contraction of the muscle undoubtedly causes compression, but it is
temporary and irrelevant. Besides, under normal conditions, the
contraction of
the internal oblique muscle presses the funiculus.
Is the
shortening and
reduction of the obliqueness of the inguinal canal not in contradiction
with
physiology? Obliqueness and the shortening of the inguinal canal do
not seem
to me to be of any great physiological relevance. I believe that it is
important
that the deep and superficial rings ought not to be aligned, but
staggered by
2-3 cm, so that the external oblique aponeurosis may effect the lower
anatomic
layers synergistically. What really counts is repairing the defense
mechanisms.In my technique the rings are never aligned, even when the
internal
oblique muscle is inserted high on the rectus sheath, because in this
case, the
neo-deep ring is created in a position that is decidedly more cranial
and medial
compared to the original one.
McVay and Anson underlined the
importance
of repairing the anatomical planes and avoiding repair between different
levels.
Do you agree? My intention was to repair physiology not anatomy.
Nevertheless, I can say that my repair is actually much more anatomical
than it
may appear to be.
Are the sutures between the two flaps of the
external
oblique aponeurosis not under traction? The external oblique
aponeurosis is
transversally very elastic. The sutures are executed in such a way as to
avoid
excessive traction and stress due to muscular contraction. The external
oblique
muscle contracts longitudinally and not transversally with respect to
the suture
line. Scarring is not impeded by the moderate tension of the suture,
also
because the aponeurosis receives a scarce blood flow and is, therefore,
not
subject to ischemia.
Is there not traction on the inguinal
ligament with
consequent risk of iatrogenic crural hernia?The risk of iatrogenic
crural
hernia is minimal even in techniques like Shouldice’s, which perform
direct
sutures along the whole inguinal ligament. Much has been written about
this
point.In my technique only the most superficial part of the inguinal
ligament is
barely involved and in no way subjected to transversal traction. I can
also say
that, since I began exploring the crural ring, I have never encountered
crural
pseudo-recurrences in my patients, while I have come across subclinical
crural
hernias in about 2% during primary hernia operations.
The
technique seems
to be complicated and rather difficult to learn and perform. Do you not
agree?
I also have the impression that this technique is not easily grasped
and
cannot really explain why. For some reason, some surgeons appear to
learn it
immediately, while others do not. It may depend on a surgeon’s ability
to free
him from traditional schemas. However, once the technique has been
understood,
it is not difficult to perform.As far " the feasibility" of the
technique is
concerned, two elements are important:
- the average operating time in my unit is about 30 minutes which indicates that the performance of the technique is not so complex
- neither recurrence nor significant complications occurred among the first patients operated availing of this technique. They represent a consistent group of patients who underwent the technique’s entire experimentation and improvement cycle. This fact corroborates the opinion that the clarity of each phase of the procedure reduces the risk of error to a minimum.
In indirect hernias, the possibility of small associated direct hernias always exists. Might a technique which does not attempt systematic repair of the posterior wall of the canal not miss them?A systematic exploration of the fossa ovalis is always carried out to ensure that subclinical crural hernias do not exist. The same is true of the posterior wall of the canal where even tiny hernias of the transversalis fascia are easily detected.I have often located very small hernias on the rectus muscle near the pubis. They are located medially and might be missed even when the posterior wall of the canal is repaired. In my technique they are occluded by the external oblique aponeurosis. However they are easily recognizable. All one has to do is look.
Does this technique not produce more postoperative pain than techniques employing tension-free meshes?I am not in a position to compare mine with tension-free mesh techniques. What I can say is that compared to other traditional methods, this technique drastically reduces postoperative pain as is shown by the limited use of painkillers: required by 35% of the patients only. Even postoperative recovery is very rapid. After one week the majority of the patients are able to move without any problem. References
AMID P.K. et al.: Femoral hernia resulting from
inguinal
herniorrhaphy: the «Plug» repair. Contemp. Surg. 39: 19-24,
1991.ASMUSSEN T.,
JENSEN F. U.: A follow-up study on recurrence after inguinal hernia
repair.
Surg. Gynecol. Obstet. 156: 198-200, 1983.
BERLINER
S.D.: Adult inguinal hernia. Pathophisiology and repair. Surg. Annu. 15:
307-329, 1983.
CANNON D.J., READ R.C.:
Metastatic
emphysema. A mechanism for acquiring inguinal herniation. Ann. Surg.
194: 270,
1981
CONNER W.T., PEACOCK E.E.: Some studies
on the
etiology of inguinal hernia. Am. J. Surg. 126: 732-736, 1973.
FASIANI G.M., CATTERINA A.: Scritti di chirurgia
erniaria.
Tipografia del Seminario di Padova, 1937.
GLASSOW
F.:
Recurrent inguinal and femoral hernia. Brit. Med. J. 1: 215-219,
1970.
GREENBURG A.G.: Revisiting the
recurrent groin
hernia. Am. J. Surg. 154: 35-40, 1987.
GUARNIERI
A. et
al.: A new technique for indirect inguinal hernia repair. Am. J. Surg.
164: 70-73, 1992GUARNIERI A., GUARNIERI F. MOSCATELLI F.: The functional
repair
of inguinal hernia. Hernia 1: 117-121, 1997
GUARNIERI
A.: La nuova chirurgia dell'ernia. Masson Milano1995
GUARNIERI A.: Procédé original de plastie "fonctionnelle" des
hernies
inguinales primaires. Chirurgie 122: 534-538, 1997
LICHTENSTEIN I.L. et al.: Pain after hernia surgery: how to
prevent it,,
how to treat it. Contemporary Surgery 33: 18-22, 1988.
LICHTENSTEIN I.L.: Herniorrhaphy. A personal experience with
6321 cases.
Am. J. Surg. 153: 553-559, 1987.
NYHUS L.M.
et al.:
Inguinal hernia. Current Problems in Surgery, Vol. XXVIII, No. 6 June
1991.
NYHUS L.M., CONDON R.E. Eds.: Hernia. Lippincott Co., Philadelphia, 1995.
PEACOCK
E.E., MADDEN J.W.: Studies on the biology and treatment of
recurrent inguinal hernia. Morphological Changes. Ann. Surg. 179:
567-571,
1974.
PIETRI P., GABRIELLI F.: Il problema
delle
recidive nella chirurgia dell'ernia inguinale. Arch. Atti Soc. Il. Chir.
87'
Congr., Torino, 1985, p. 59-79.
POSTLETHWAIT
R.W.:
Recurrent inguinal hernia. Ann. Surg. 202: 777-779, 1985.
READ R.C., McLEOD P.C.: Influence of a relaxing incision on
suture
tension in Bassini's and McVays repairs. Arch. Surg. 116: 440-445, 1981.
READ R.C.: Attenuation of the rectus Sheath in
inguinal
herniation. Am. J. Surg. 120: 610-614, 1970.
READ
R.C.:
Properitoneal herniorrhaphy,: a historical review. World J. Surg. 13:
532-539,
1989.
READ R.C.: YODER G.: Recent trends in
the
management of incisional herniation. Arch. Surg. 124: 485-488, 1989
RIVES J., NICAISE H.: Hernies inguinales.
Encyclopédie
Medico-Chirurgicale, vol. 1 App. digestif 40105-40110, 1978.SCHUMPELICK
V. et
al.: Repair of recurrent inguinal hernia. Tactics, technic and results.
Chirurg
61: 526-529, 1990.
SHANDALAKIS J.E. et al.:
Surgical
anatomy of the inguinal area. World J. Surg. 13: 490-498, 1989.
SHULMANN A.G. el al. The «Plug» repair of 1402 recurrent
inguinal
hernias. 20- Years experience. Arch. Surg. 125: 265-267, 1990.
STOPPA R. et al.: Complications des réparations prothétiques
des hernies
de l'aine. Chirurgie, 113: 195-204, 1987.
THOMAS
S.M.,
PEYTON BARNES J: Recurrent inguinal hernia in relation to
ideal body weight. Surg. Gynecol. Obstet. 170:
510-512,
1990.
WAGH P.V., READ R.C.: Defective
collagen
synthesis in inguinal herniation. Am. J. Surg.
124:
819-822, 1972.
YOUNG D.V.: Comparison of
local spinal
and general anesthesia for inguinal herniorrhaphy,. Am. J. Surg. 153:
560-563,
1987.
APPENDIX
12 THE "SANDWICH" TECHNIQUE IN INCISIONAL HERNIAS
This method was published by me in "Atti della Societa' Italiana di Chirurgia" in 1988, and later, in 1991, it appeared in the World Journal of Surgery as an original method by Matapurkar. This a technique particularly suited for the treatment of multiple incisional hernias and cases where the hernial defect is rigid due to fibrosis of the wall. Normally the mesh is a must but it means creating contact between the mesh, the bowel and subcutaneous tissue, with greater consequent risk of complication. The "Sandwich" technique permits that the mesh is separated from them, because the prosthesis is located between the two flaps created by the division of the hernial sac into two parts.
Incisions
Excision of the skin
and
subcutaneous tissue is generally preferred; this is performed by
carrying out
two incisions externally to the neck of the hernia sac, on scarfree
tissue.
In this way, the scar area which surrounds the sac is avoided and the
fascial
layer is easily reached. Following this level, the hernial defect and
the sac
,which is neither resected nor opened until it has been isolated
completely, are
easily reached.
Treatment of the sac
In
incisional hernias, the sac is a continuation of the aponeurotic
muscular plane
from which it may not be separated. As stated, the sac should not be
resected;
it is isolated as far as the neck and then divided longitudinally into
two
halves, perpendicular to the wall (Fig. 12.1). The adhesions are eliminated for a wide tract of the
parietal
peritoneum and the contents of the sac is pushed into the abdomen.
The longitudinal division of the sac creates two flaps (Fig. 12.1A).The free edge of the flap on the surgeon’s side of the patient is positioned inside the hernial defect and is sutured to the parietal peritoneum along the entire free hemicircumference facing the insertion (Fig. 12.2A). The suture continues using synthetic absorbable thread, and is carried out at the greatest possible distance from the neck and includes, even if only partially, the muscular plane above it.A polypropylene mesh is shaped to the outline of the hernial defect, exceeding its diameter by 4 cm.. The hemicircumference on the side opposite the surgeon is fixed to the parietal peritoneum with a non-absorbable continuous suture concentric to the previous one.The other hemicircumference is sutured to the premuscular fascial layer (externally to the hernial defect).A third continuous suture is carried out with non-absorbable thread to anchor the mesh to the edge of the hernia defect (Fig. 12.2B).The second flap of the hernial sac (opposite the surgeon) is pulled over the mesh, so that to cover it completely (Fig. 12.2 C). The free hemicircumference is fixed on outside of the mesh at fascial layer, using the absorbable thread employed for the first suture.